Personalized Proteomics in Proliferative Vitreoretinopathy Implicate Hematopoietic Cell Recruitment and mTOR as a Therapeutic Target
- PMID: 29246578
- PMCID: PMC5805631
- DOI: 10.1016/j.ajo.2017.11.025
Personalized Proteomics in Proliferative Vitreoretinopathy Implicate Hematopoietic Cell Recruitment and mTOR as a Therapeutic Target
Abstract
Purpose: To profile vitreous cytokine expression of proliferative vitreoretinopathy (PVR) patients.
Design: Case-control study.
Methods: Liquid biopsies were collected from 2 groups: control subjects (n = 3) undergoing pars plana vitrectomy to remove an epiretinal membrane (ERM), and test subjects (n = 7) with varying degrees of PVR. A high-throughput cytokine screen measured expression of 200 cytokines. Cytokine expression patterns were prospectively validated in separate cohorts of control patients and those with PVR-A, PVR-B, and PVR-C (n = 10 for each group). Expression changes were evaluated by analysis of variance (significant P value < .05), hierarchical cluster algorithm, and pathway analysis, to identify candidate pathways for prospective studies.
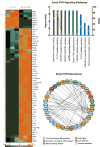
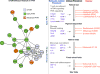
Results: In PVR vitreous, 29 cytokines were upregulated compared to controls. Early PVR vitreous showed upregulation of T-cell markers, profibrotic cytokines, and cytokines downstream of mTOR activation (IL-2, IL-6, and IL-13), whereas in late PVR vitreous, cytokines driving monocyte responses and stem-cell recruitment (SDF-1) prevailed. Prospective validation confirmed the differential expression of specific cytokines from PVR-A to C.
Conclusions: Early PVR is characterized by activation of T cells and mTOR signaling, whereas advanced PVR is characterized by a chronic monocyte response. PVR might be treated by rational repositioning of existing drugs that target mTOR and IL-6. Our analysis demonstrates that successful therapeutic intervention will be highly dependent on the specific therapeutic target and the stage of PVR. This study provides insights into cytokines that will serve as biomarkers and therapeutic targets. These biomarkers will help design clinical trials that intervene at appropriate times.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Disclosures: None reported.
Figures



References
-
- Tseng W, Cortez RT, Ramirez G, Stinnett S, Jaffe GJ. Prevalence and risk factors for proliferative vitreoretinopathy in eyes with rhegmatogenous retinal detachment but no previous vitreoretinal surgery. Am J Ophthalmol. 2004;137(6):1105–1115. - PubMed
-
- Scott IU, Flynn HW, Jr, Murray TG, Feuer WJ, Perfluoron study g Outcomes of surgery for retinal detachment associated with proliferative vitreoretinopathy using perfluoro-n-octane: a multicenter study. Am J Ophthalmol. 2003;136(3):454–463. - PubMed
-
- Machemer R, Aaberg TM, Freeman HM, Irvine AR, Lean JS, Michels RM. An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol. 1991;112(2):159–165. - PubMed
-
- Pastor JC, Rojas J, Pastor-Idoate S, Di Lauro S, Gonzalez-Buendia L, Delgado-Tirado S. Proliferative vitreoretinopathy: A new concept of disease pathogenesis and practical consequences. Prog Retin Eye Res. 2016;51:125–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

