Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial
- PMID: 29246803
- PMCID: PMC6027999
- DOI: 10.1016/S1470-2045(17)30909-9
Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial
Abstract
Background: Therapy targeting Bruton's tyrosine kinase (BTK) with ibrutinib has transformed the treatment of chronic lymphocytic leukaemia. However, patients who are refractory to or relapse after ibrutinib therapy have poor outcomes. Venetoclax is a selective, orally bioavailable inhibitor of BCL-2 active in previously treated patients with relapsed or refractory chronic lymphocytic leukaemia. In this study, we assessed the activity and safety of venetoclax in patients with chronic lymphocytic leukaemia who are refractory to or relapse during or after ibrutinib therapy.
Methods: In this interim analysis of a multicentre, open-label, non-randomised, phase 2 trial, we enrolled patients aged 18 years or older with a documented diagnosis of chronic lymphocytic leukaemia according to the 2008 International Workshop on Chronic Lymphocytic Leukemia (IWCLL) criteria and an Eastern Cooperative Oncology Group performance score of 2 or lower. All patients had relapsed or refractory disease after previous treatment with a BCR signalling pathway inhibitor. All patients were screened for Richter's transformation and cases confirmed by biopsy were excluded. Eligible patients received oral venetoclax, starting at 20 mg per day with stepwise dose ramp-up over 5 weeks to 400 mg per day. Patients with rapidly progressing disease received an accelerated dosing schedule (to 400 mg per day by week 3). The primary endpoint was overall response, defined as the proportion of patients with an overall response per investigator's assessment according to IWCLL criteria. All patients who received at least one dose of venetoclax were included in the activity and safety analyses. This study is ongoing; data for this interim analysis were collected per regulatory agencies' request as of June 30, 2017. This trial is registered with ClinicalTrials.gov, number NCT02141282.
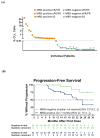
Findings: Between September, 2014, and November, 2016, 127 previously treated patients with relapsed or refractory chronic lymphocytic leukaemia were enrolled from 15 sites across the USA. 91 patients had received ibrutinib as the last BCR inhibitor therapy before enrolment, 43 of whom were enrolled in the main cohort and 48 in the expansion cohort recruited later after a protocol amendment. At the time of analysis, the median follow-up was 14 months (IQR 8-18) for all 91 patients, 19 months (9-27) for the main cohort, and 12 months (8-15) for the expansion cohort. 59 (65%, 95% CI 53-74) of 91 patients had an overall response, including 30 (70%, 54-83) of 43 patients in the main cohort and 29 (60%, 43-72) of 48 patients in the expansion cohort. The most common treatment-emergent grade 3 or 4 adverse events were neutropenia (46 [51%] of 91 patients), thrombocytopenia (26 [29%]), anaemia (26 [29%]), decreased white blood cell count (17 [19%]), and decreased lymphocyte count (14 [15%]). 17 (19%) of 91 patients died, including seven because of disease progression. No treatment-related deaths occurred.
Interpretation: The results of this interim analysis show that venetoclax has durable clinical activity and favourable tolerability in patients with relapsed or refractory chronic lymphocytic leukaemia whose disease progressed during or after discontinutation of ibrutinib therapy. The durability of response to venetoclax will be assessed in the final analysis in 2019.
Funding: AbbVie, Genentech.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


Comment in
-
Venetoclax: a chance for patients with chronic lymphocytic leukaemia previously treated with ibrutinib.Lancet Oncol. 2018 Jan;19(1):7-8. doi: 10.1016/S1470-2045(17)30910-5. Epub 2017 Dec 12. Lancet Oncol. 2018. PMID: 29246804 No abstract available.
References
-
- Jain N, O’Brien S. Targeted therapies for CLL: Practical issues with the changing treatment paradigm. Blood Rev. 2016;30:233–44. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

