Global Pulmonary Vascular Remodeling in Pulmonary Hypertension Associated With Heart Failure and Preserved or Reduced Ejection Fraction
- PMID: 29246894
- PMCID: PMC5915920
- DOI: 10.1161/CIRCULATIONAHA.117.031608
Global Pulmonary Vascular Remodeling in Pulmonary Hypertension Associated With Heart Failure and Preserved or Reduced Ejection Fraction
Abstract
Background: We hypothesized that pulmonary venous hypertension in heart failure (HF) leads to predominate remodeling of pulmonary veins and that the severity of venous remodeling is associated with the severity of pulmonary hypertension (PH) in HF.
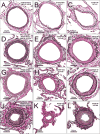
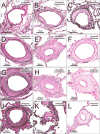
Methods: Patients with HF (n=108; 53 preserved and 55 reduced ejection fraction) with PH (HF-PH; pulmonary artery systolic pressure [PASP] ≥40 mm Hg) were compared to normal controls (n=12) and patients with primary pulmonary veno-occlusive disease (PVOD; n=17). In lung specimens from autopsy (control, HF-PH, and 7 PVOD) or surgery (10 PVOD), quantitative histomorphometry was performed in all analyzable arteries (n=4949), veins (n=7630), and small indeterminate vessels (IV; n=2168) to define percent medial thickness (arteries) and percent intimal thickness (%IT) (arteries, veins, and IV) relative to external diameter.
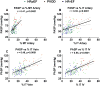
Results: The average arterial percent medial thickness (control, 6.9; HF-PH, 11.0; PVOD, 15.0), arterial %IT (control, 4.9; HF-PH, 14.9; PVOD, 31.1), venous %IT (control, 14.0; HF-PH, 24.9; PVOD, 43.9), and IV %IT (control, 10.6; HF-PH, 25.8; PVOD, 50.0) in HF-PH were higher than controls (P<0.0001 for all) but lower than PVOD (P≤0.005 for all). PASP (mm Hg) was lower in HF-PH (median, 59 [interquartile range, 50-70]) than in PVOD (median, 91 [interquartile range, 82-103]). PASP correlated with arterial percent medial thickness (r=0.41) and arterial %IT (r=0.35) but more strongly with venous %IT (r=0.49) and IV %IT (r=0.55) (P<0.0001 for all). Associations between PASP and venous or IV %IT remained significant after adjusting for arterial percent medial thickness and %IT and did not vary by HF type. In patients with right heart catheterization (30 HF-PH, 14 PVOD), similar associations between the transpulmonary gradient and pulmonary vascular remodeling existed, with numerically stronger associations for venous and IV %IT. Although the PASP was slightly higher in patients with HF-PH with right ventricular dysfunction, pulmonary vascular remodeling was not more severe. Pulmonary vascular remodeling severity was associated with reductions in the diffusing capacity of the lungs.
Conclusions: In HF, PH is associated with global pulmonary vascular remodeling, but the severity of PH correlates most strongly with venous and small IV intimal thickening, similar to the pattern observed in PVOD. These findings expand our understanding of the pathobiology of PH in HF.
Keywords: diffusing capacity of the lungs for carbon monoxide; heart failure; heart failure with preserved ejection fraction; pulmonary function; pulmonary hypertension; right ventricle.
© 2017 American Heart Association, Inc.
Figures



Comment in
-
Pulmonary Venous Remodeling in Pulmonary Hypertension: The Veins Take Center Stage.Circulation. 2018 Apr 24;137(17):1811-1813. doi: 10.1161/CIRCULATIONAHA.118.033013. Circulation. 2018. PMID: 29685930 Free PMC article. No abstract available.
References
-
- Tuder RM, Stacher E, Robinson J, Kumar R, Graham BB. Pathology of pulmonary hypertension. Clin Chest Med. 2013;34:639–650. - PubMed
-
- Montani D, Lau EM, Dorfmuller P, Girerd B, Jais X, Savale L, Perros F, Nossent E, Garcia G, Parent F, Fadel E, Soubrier F, Sitbon O, Simonneau G, Humbert M. Pulmonary veno-occlusive disease. Eur Respir J. 2016;47:1518–1534. - PubMed
-
- Galie N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, McGoon MD, McLaughlin VV, Preston IR, Rubin LJ, Sandoval J, Seeger W, Keogh A. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62:D60–72. - PubMed
-
- Montani D, O'Callaghan DS, Savale L, Jais X, Yaici A, Maitre S, Dorfmuller P, Sitbon O, Simonneau G, Humbert M. Pulmonary veno-occlusive disease: recent progress and current challenges. Respir Med. 2010;104(1):S23–32. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

