Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria
- PMID: 29246946
- PMCID: PMC5759965
- DOI: 10.1126/sciimmunol.aao1314
Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria
Abstract
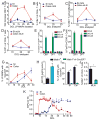
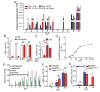
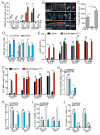
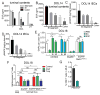
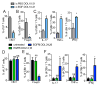
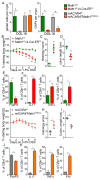
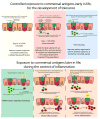
We have a mutually beneficial relationship with the trillions of microorganisms inhabiting our gastrointestinal tract. However, maintaining this relationship requires recognizing these organisms as affable and restraining inflammatory responses to these organisms when encountered in hostile settings. How and when the immune system develops tolerance to our gut microbial members is not well understood. We identify a specific preweaning interval in which gut microbial antigens are encountered by the immune system to induce antigen-specific tolerance to gut bacteria. For some bacterial taxa, physiologic encounters with the immune system are restricted to this interval, despite abundance of these taxa in the gut lumen at later times outside this interval. Antigen-specific tolerance to gut bacteria induced during this preweaning interval is stable and maintained even if these taxa are encountered later in life in an inflammatory setting. However, inhibiting microbial antigen encounter during this interval or extending these encounters beyond the normal interval results in a failure to induce tolerance and robust antigen-specific effector responses to gut bacteria upon reencounter in an inflammatory setting. Thus, we have identified a defined preweaning interval critical for developing tolerance to gut bacteria and maintaining the mutually beneficial relationship with our gut microbiota.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures

Comment in
-
GAPs in early life facilitate immune tolerance.Sci Immunol. 2017 Dec 15;2(18):eaar2465. doi: 10.1126/sciimmunol.aar2465. Sci Immunol. 2017. PMID: 29246947
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

