Mannose receptor high, M2 dermal macrophages mediate nonhealing Leishmania major infection in a Th1 immune environment
- PMID: 29247046
- PMCID: PMC5748861
- DOI: 10.1084/jem.20171389
Mannose receptor high, M2 dermal macrophages mediate nonhealing Leishmania major infection in a Th1 immune environment
Abstract
The origin and functional specialization of dermal macrophages in cutaneous infections have been little studied. In this paper, we show that a strain of Leishmania major (L. major Seidman [LmSd]) that produces nonhealing cutaneous lesions in conventionally resistant C57BL/6 mice was more efficiently taken up by M2-polarized bone marrow (BM)-derived macrophages (BMDMs) in vitro and by mannose receptor (MR)hi dermal macrophages in vivo compared with a healing strain (L. major Friedlin V1). Both in steady and in T helper type 1 (Th1) cell-driven inflammatory states, the MRhi dermal macrophages showed M2 characteristics. The dermal macrophages were radio resistant and not replaced by monocytes or adult BM-derived cells during infection, but were locally maintained by IL-4 and IL-10. Notably, the favored infection of M2 BMDMs by LmSd in vitro was MR dependent, and genetic deletion of MR or selective depletion of MRhi dermal macrophages by anti-CSF-1 receptor antibody reversed the nonhealing phenotype. We conclude that embryonic-derived, MRhi dermal macrophages are permissive for parasite growth even in a strong Th1-immune environment, and the preferential infection of these cells plays a crucial role in the severity of cutaneous disease.
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Figures

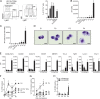
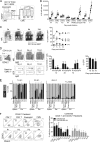

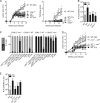


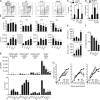

Comment in
-
Mannose Receptor and the Mystery of Nonhealing Leishmania major Infection.Trends Parasitol. 2018 May;34(5):354-356. doi: 10.1016/j.pt.2018.03.006. Epub 2018 Apr 10. Trends Parasitol. 2018. PMID: 29650366
Similar articles
-
The Nlrp3 inflammasome, IL-1β, and neutrophil recruitment are required for susceptibility to a nonhealing strain of Leishmania major in C57BL/6 mice.Eur J Immunol. 2016 Apr;46(4):897-911. doi: 10.1002/eji.201546015. Epub 2016 Jan 20. Eur J Immunol. 2016. PMID: 26689285 Free PMC article.
-
Nonhealing infection despite Th1 polarization produced by a strain of Leishmania major in C57BL/6 mice.J Immunol. 2005 Mar 1;174(5):2934-41. doi: 10.4049/jimmunol.174.5.2934. J Immunol. 2005. PMID: 15728505
-
Mannose Receptor and the Mystery of Nonhealing Leishmania major Infection.Trends Parasitol. 2018 May;34(5):354-356. doi: 10.1016/j.pt.2018.03.006. Epub 2018 Apr 10. Trends Parasitol. 2018. PMID: 29650366
-
Requirements for Th1-dependent immunity against infection with Leishmania major.Microbes Infect. 2004 Oct;6(12):1102-9. doi: 10.1016/j.micinf.2004.05.024. Microbes Infect. 2004. PMID: 15380780 Review.
-
T(H)1 cells control themselves by producing interleukin-10.Nat Rev Immunol. 2007 Jun;7(6):425-8. doi: 10.1038/nri2097. Nat Rev Immunol. 2007. PMID: 17525751 Review.
Cited by
-
Low-level Cxcr4-haploinsufficient HSC engraftment is sufficient to correct leukopenia in WHIM syndrome mice.JCI Insight. 2019 Dec 19;4(24):e132140. doi: 10.1172/jci.insight.132140. JCI Insight. 2019. PMID: 31687976 Free PMC article.
-
Immunometabolism at the interface between macrophages and pathogens.Nat Rev Immunol. 2019 May;19(5):291-304. doi: 10.1038/s41577-019-0124-9. Nat Rev Immunol. 2019. PMID: 30679807 Free PMC article. Review.
-
Field-Deployable Treatments For Leishmaniasis: Intrinsic Challenges, Recent Developments and Next Steps.Res Rep Trop Med. 2023 Jul 20;14:61-85. doi: 10.2147/RRTM.S392606. eCollection 2023. Res Rep Trop Med. 2023. PMID: 37492219 Free PMC article. Review.
-
Resilience of dermis resident macrophages to inflammatory challenges.Exp Mol Med. 2024 Oct;56(10):2105-2112. doi: 10.1038/s12276-024-01313-z. Epub 2024 Oct 1. Exp Mol Med. 2024. PMID: 39349826 Free PMC article. Review.
-
Modulation of Macrophage Immunometabolism: A New Approach to Fight Infections.Front Immunol. 2022 Jan 26;13:780839. doi: 10.3389/fimmu.2022.780839. eCollection 2022. Front Immunol. 2022. PMID: 35154105 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

