Voltage modulates halothane-triggered Ca2+ release in malignant hyperthermia-susceptible muscle
- PMID: 29247050
- PMCID: PMC5749113
- DOI: 10.1085/jgp.201711864
Voltage modulates halothane-triggered Ca2+ release in malignant hyperthermia-susceptible muscle
Abstract
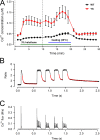
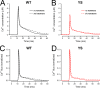
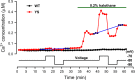
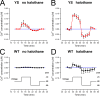
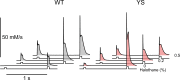
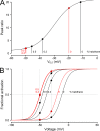
Malignant hyperthermia (MH) is a fatal hypermetabolic state that may occur during general anesthesia in susceptible individuals. It is often caused by mutations in the ryanodine receptor RyR1 that favor drug-induced release of Ca2+ from the sarcoplasmic reticulum. Here, knowing that membrane depolarization triggers Ca2+ release in normal muscle function, we study the cross-influence of membrane potential and anesthetic drugs on Ca2+ release. We used short single muscle fibers of knock-in mice heterozygous for the RyR1 mutation Y524S combined with microfluorimetry to measure intracellular Ca2+ signals. Halothane, a volatile anesthetic used in contracture testing for MH susceptibility, was equilibrated with the solution superfusing the cells by means of a vaporizer system. In the range 0.2 to 3%, the drug causes significantly larger elevations of free myoplasmic [Ca2+] in mutant (YS) compared with wild-type (WT) fibers. Action potential-induced Ca2+ signals exhibit a slowing of their time course of relaxation that can be attributed to a component of delayed Ca2+ release turnoff. In further experiments, we applied halothane to single fibers that were voltage-clamped using two intracellular microelectrodes and studied the effect of small (10-mV) deviations from the holding potential (-80 mV). Untreated WT fibers show essentially no changes in [Ca2+], whereas the Ca2+ level of YS fibers increases and decreases on depolarization and hyperpolarization, respectively. The drug causes a significant enhancement of this response. Depolarizing pulses reveal a substantial negative shift in the voltage dependence of activation of Ca2+ release. This behavior likely results from the allosteric coupling between RyR1 and its transverse tubular voltage sensor. We conclude that the binding of halothane to RyR1 alters the voltage dependence of Ca2+ release in MH-susceptible muscle fibers such that the resting membrane potential becomes a decisive factor for the efficiency of the drug to trigger Ca2+ release.
© 2018 Zullo et al.
Figures

References
-
- Adnet P.J., Krivosic-Horber R.M., Adamantidis M.M., Haudecoeur G., Reyford H.G., and Dupuis B.A.. 1992. Is resting membrane potential a possible indicator of viability of muscle bundles used in the in vitro caffeine contracture test? Anesth. Analg. 74:105–111. 10.1213/00000539-199201000-00017 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

