LRX Proteins Play a Crucial Role in Pollen Grain and Pollen Tube Cell Wall Development
- PMID: 29247121
- PMCID: PMC5841697
- DOI: 10.1104/pp.17.01374
LRX Proteins Play a Crucial Role in Pollen Grain and Pollen Tube Cell Wall Development
Abstract
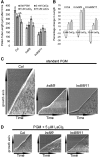
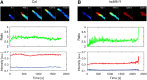
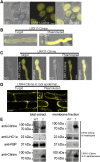
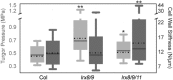
Leu-rich repeat extensins (LRXs) are chimeric proteins containing an N-terminal Leu-rich repeat (LRR) and a C-terminal extensin domain. LRXs are involved in cell wall formation in vegetative tissues and required for plant growth. However, the nature of their role in these cellular processes remains to be elucidated. Here, we used a combination of molecular techniques, light microscopy, and transmission electron microscopy to characterize mutants of pollen-expressed LRXs in Arabidopsis (Arabidopsisthaliana). Mutations in multiple pollen-expressed lrx genes cause severe defects in pollen germination and pollen tube growth, resulting in a reduced seed set. Physiological experiments demonstrate that manipulating Ca2+ availability partially suppresses the pollen tube growth defects, suggesting that LRX proteins influence Ca2+-related processes. Furthermore, we show that LRX protein localizes to the cell wall, and its LRR-domain (which likely mediates protein-protein interactions) is associated with the plasma membrane. Mechanical analyses by cellular force microscopy and finite element method-based modeling revealed significant changes in the material properties of the cell wall and the fine-tuning of cellular biophysical parameters in the mutants compared to the wild type. The results indicate that LRX proteins might play a role in cell wall-plasma membrane communication, influencing cell wall formation and cellular mechanics.
© 2018 American Society of Plant Biologists. All Rights Reserved.
Figures



Comment in
-
Coordinating Cell Walls and Cell Growth: A Role for LRX Extensin Chimeras.Plant Physiol. 2018 Mar;176(3):1890-1891. doi: 10.1104/pp.18.00084. Plant Physiol. 2018. PMID: 29630498 Free PMC article. No abstract available.
References
-
- Alexander MP. (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44: 117–122 - PubMed
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Bai L, Zhang G, Zhou Y, Zhang Z, Wang W, Du Y, Wu Z, Song CP (2009) Plasma membrane-associated proline-rich extensin-like receptor kinase 4, a novel regulator of Ca signalling, is required for abscisic acid responses in Arabidopsis thaliana. Plant J 60: 314–327 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

