Increasing the permeability of Escherichia coli using MAC13243
- PMID: 29247166
- PMCID: PMC5732295
- DOI: 10.1038/s41598-017-17772-6
Increasing the permeability of Escherichia coli using MAC13243
Abstract
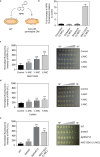
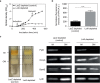
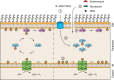
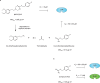
The outer membrane of gram-negative bacteria is a permeability barrier that prevents the efficient uptake of molecules with large scaffolds. As a consequence, a number of antibiotic classes are ineffective against gram-negative strains. Herein we carried out a high throughput screen for small molecules that make the outer membrane of Escherichia coli more permeable. We identified MAC13243, an inhibitor of the periplasmic chaperone LolA that traffics lipoproteins from the inner to the outer membrane. We observed that cells were (1) more permeable to the fluorescent probe 1-N-phenylnapthylamine, and (2) more susceptible to large-scaffold antibiotics when sub-inhibitory concentrations of MAC13243 were used. To exclude the possibility that the permeability was caused by an off-target effect, we genetically reconstructed the MAC13243-phenotype by depleting LolA levels using the CRISPRi system.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Degradation of MAC13243 and studies of the interaction of resulting thiourea compounds with the lipoprotein targeting chaperone LolA.Bioorg Med Chem Lett. 2013 Apr 15;23(8):2426-31. doi: 10.1016/j.bmcl.2013.02.005. Epub 2013 Feb 13. Bioorg Med Chem Lett. 2013. PMID: 23473681
-
Genetic analysis reveals a robust and hierarchical recruitment of the LolA chaperone to the LolCDE lipoprotein transporter.mBio. 2024 Feb 14;15(2):e0303923. doi: 10.1128/mbio.03039-23. Epub 2024 Jan 9. mBio. 2024. PMID: 38193657 Free PMC article.
-
A small-molecule inhibitor of BamA impervious to efflux and the outer membrane permeability barrier.Proc Natl Acad Sci U S A. 2019 Oct 22;116(43):21748-21757. doi: 10.1073/pnas.1912345116. Epub 2019 Oct 7. Proc Natl Acad Sci U S A. 2019. PMID: 31591200 Free PMC article.
-
ABC transporters involved in the biogenesis of the outer membrane in gram-negative bacteria.Biosci Biotechnol Biochem. 2011;75(6):1044-54. doi: 10.1271/bbb.110115. Epub 2011 Jun 13. Biosci Biotechnol Biochem. 2011. PMID: 21670534 Review.
-
Sorting of lipoproteins to the outer membrane in E. coli.Biochim Biophys Acta. 2004 Nov 11;1694(1-3):IN1-9. Biochim Biophys Acta. 2004. PMID: 15672528 Review.
Cited by
-
Chemical genetic approaches for the discovery of bacterial cell wall inhibitors.RSC Med Chem. 2023 Aug 30;14(11):2125-2154. doi: 10.1039/d3md00143a. eCollection 2023 Nov 15. RSC Med Chem. 2023. PMID: 37974958 Free PMC article. Review.
-
Antibacterial Efficacy and Mechanisms of Action of a Novel Beta-Defensin from Snakehead Murrel, Channa striata.Probiotics Antimicrob Proteins. 2024 Jul 4. doi: 10.1007/s12602-024-10307-2. Online ahead of print. Probiotics Antimicrob Proteins. 2024. PMID: 38963507
-
The carRS-ompV-virK operon of Vibrio cholerae senses antimicrobial peptides and activates the expression of multiple resistance systems.Sci Rep. 2025 Apr 21;15(1):13686. doi: 10.1038/s41598-025-98217-3. Sci Rep. 2025. PMID: 40258937 Free PMC article.
-
Mode of action of lipoprotein modification enzymes-Novel antibacterial targets.Mol Microbiol. 2021 Mar;115(3):356-365. doi: 10.1111/mmi.14610. Epub 2020 Oct 12. Mol Microbiol. 2021. PMID: 32979868 Free PMC article. Review.
-
Ethanol in Combination with Oxidative Stress Significantly Impacts Mycobacterial Physiology.J Bacteriol. 2020 Nov 4;202(23):e00222-20. doi: 10.1128/JB.00222-20. Print 2020 Nov 4. J Bacteriol. 2020. PMID: 32928928 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

