Changes in hyaluronan deposition in the rat myenteric plexus after experimentally-induced colitis
- PMID: 29247178
- PMCID: PMC5732300
- DOI: 10.1038/s41598-017-18020-7
Changes in hyaluronan deposition in the rat myenteric plexus after experimentally-induced colitis
Abstract
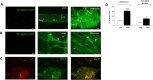
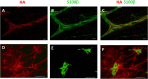
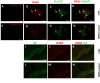
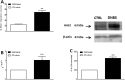
Myenteric plexus alterations hamper gastrointestinal motor function during intestinal inflammation. Hyaluronan (HA), an extracellular matrix glycosaminoglycan involved in inflammatory responses, may play a role in this process. In the colon of control rats, HA-binding protein (HABP), was detected in myenteric neuron soma, perineuronal space and ganglia surfaces. Prominent hyaluronan synthase 2 (HAS2) staining was found in myenteric neuron cytoplasm, suggesting that myenteric neurons produce HA. In the myenteric plexus of rats with 2, 4-dinitrobenzene sulfonic (DNBS)-induced colitis HABP staining was altered in the perineuronal space, while both HABP staining and HA levels increased in the muscularis propria. HAS2 immunopositive myenteric neurons and HAS2 mRNA and protein levels also increased. Overall, these observations suggest that inflammation alters HA distribution and levels in the gut neuromuscular compartment. Such changes may contribute to alterations in the myenteric plexus.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Involvement of hyaluronan in the adaptive changes of the rat small intestine neuromuscular function after ischemia/reperfusion injury.Sci Rep. 2020 Jul 13;10(1):11521. doi: 10.1038/s41598-020-67876-9. Sci Rep. 2020. PMID: 32661417 Free PMC article.
-
Myenteric plexus injury and apoptosis in experimental colitis.Auton Neurosci. 2005 Jan 15;117(1):41-53. doi: 10.1016/j.autneu.2004.10.006. Auton Neurosci. 2005. PMID: 15620569
-
Selective up-regulation of NMDA-NR1 receptor expression in myenteric plexus after TNBS induced colitis in rats.Mol Pain. 2006 Jan 17;2:3. doi: 10.1186/1744-8069-2-3. Mol Pain. 2006. PMID: 16417630 Free PMC article.
-
Cell Energy Metabolism and Hyaluronan Synthesis.J Histochem Cytochem. 2021 Jan;69(1):35-47. doi: 10.1369/0022155420929772. Epub 2020 Jul 6. J Histochem Cytochem. 2021. PMID: 32623953 Free PMC article. Review.
-
Role of hyaluronan in atherosclerosis: Current knowledge and open questions.Matrix Biol. 2019 May;78-79:324-336. doi: 10.1016/j.matbio.2018.03.003. Epub 2018 Mar 3. Matrix Biol. 2019. PMID: 29510229 Review.
Cited by
-
Tryptophan Metabolites Along the Microbiota-Gut-Brain Axis: An Interkingdom Communication System Influencing the Gut in Health and Disease.Int J Tryptophan Res. 2020 Jun 11;13:1178646920928984. doi: 10.1177/1178646920928984. eCollection 2020. Int J Tryptophan Res. 2020. PMID: 32577079 Free PMC article. Review.
-
Effect of partial substitution of fishmeal with insect meal (Hermetia illucens) on gut neuromuscular function in Gilthead sea bream (Sparus aurata).Sci Rep. 2021 Nov 8;11(1):21788. doi: 10.1038/s41598-021-01242-1. Sci Rep. 2021. PMID: 34750477 Free PMC article.
-
Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis.Int J Mol Sci. 2019 Mar 25;20(6):1482. doi: 10.3390/ijms20061482. Int J Mol Sci. 2019. PMID: 30934533 Free PMC article. Review.
-
Hyaluronan Regulates Neuronal and Immune Function in the Rat Small Intestine and Colonic Microbiota after Ischemic/Reperfusion Injury.Cells. 2022 Oct 25;11(21):3370. doi: 10.3390/cells11213370. Cells. 2022. PMID: 36359764 Free PMC article.
-
Hyaluronan Accelerates Intestinal Mucosal Healing through Interaction with TSG-6.Cells. 2019 Sep 12;8(9):1074. doi: 10.3390/cells8091074. Cells. 2019. PMID: 31547322 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

