Hepatocytic expression of human sodium-taurocholate cotransporting polypeptide enables hepatitis B virus infection of macaques
- PMID: 29247188
- PMCID: PMC5732258
- DOI: 10.1038/s41467-017-01953-y
Hepatocytic expression of human sodium-taurocholate cotransporting polypeptide enables hepatitis B virus infection of macaques
Abstract
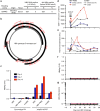
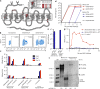
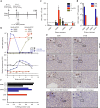
Hepatitis B virus (HBV) is a major global health concern, and the development of curative therapeutics is urgently needed. Such efforts are impeded by the lack of a physiologically relevant, pre-clinical animal model of HBV infection. Here, we report that expression of the HBV entry receptor, human sodium-taurocholate cotransporting polypeptide (hNTCP), on macaque primary hepatocytes facilitates HBV infection in vitro, where all replicative intermediates including covalently closed circular DNA (cccDNA) are present. Furthermore, viral vector-mediated expression of hNTCP on hepatocytes in vivo renders rhesus macaques permissive to HBV infection. These in vivo macaque HBV infections are characterized by longitudinal HBV DNA in serum, and detection of HBV DNA, RNA, and HBV core antigen (HBcAg) in hepatocytes. Together, these results show that expressing hNTCP on macaque hepatocytes renders them susceptible to HBV infection, thereby establishing a physiologically relevant model of HBV infection to study immune clearance and test therapeutic and curative approaches.
Conflict of interest statement
Oregon Health and Science University (OHSU), Dr. Sacha, and Dr. Burwitz have a financial interest in Vir Biotechnology, Inc., a company that may have a financial interest in the results of this research and technology. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU. The remaining authors declare no competing financial interests.
Figures
References
-
- Ely A, Arbuthnot P. Differing prospects for the future of using gene therapy to treat infections with hepatitis B virus and hepatitis C virus. Discov. Med. 2015;20:137–143. - PubMed
-
- Andersson, K. L. & Chung, R. T. in Monitoring during and after antiviral therapy for hepatitis B. Vol. 49 (ed. Hoofnagle, J. H.) 166–S173 (Wiley Subscription Services, Inc., A Wiley Company, 2009).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

