Cell shape information is transduced through tension-independent mechanisms
- PMID: 29247198
- PMCID: PMC5732205
- DOI: 10.1038/s41467-017-02218-4
Cell shape information is transduced through tension-independent mechanisms
Abstract
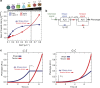
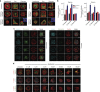
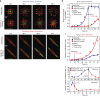
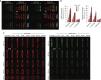
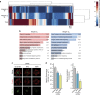
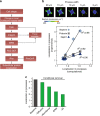
The shape of a cell within tissues can represent the history of chemical and physical signals that it encounters, but can information from cell shape regulate cellular phenotype independently? Using optimal control theory to constrain reaction-diffusion schemes that are dependent on different surface-to-volume relationships, we find that information from cell shape can be resolved from mechanical signals. We used microfabricated 3-D biomimetic chips to validate predictions that shape-sensing occurs in a tension-independent manner through integrin β3 signaling pathway in human kidney podocytes and smooth muscle cells. Differential proteomics and functional ablation assays indicate that integrin β3 is critical in transduction of shape signals through ezrin-radixin-moesin (ERM) family. We used experimentally determined diffusion coefficients and experimentally validated simulations to show that shape sensing is an emergent cellular property enabled by multiple molecular characteristics of integrin β3. We conclude that 3-D cell shape information, transduced through tension-independent mechanisms, can regulate phenotype.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Inhibition of cell adhesion by phosphorylated Ezrin/Radixin/Moesin.Cell Adh Migr. 2015;9(6):502-12. doi: 10.1080/19336918.2015.1113366. Cell Adh Migr. 2015. PMID: 26555866 Free PMC article.
-
Cyclic AMP prevents decrease of phosphorylated ezrin/radixin/moesin and chloride intracellular channel 5 expressions in injured podocytes.Clin Exp Nephrol. 2015 Dec;19(6):1000-6. doi: 10.1007/s10157-015-1102-6. Epub 2015 Mar 1. Clin Exp Nephrol. 2015. PMID: 25725994
-
Both CLIC4 and CLIC5A activate ERM proteins in glomerular endothelium.Am J Physiol Renal Physiol. 2016 Nov 1;311(5):F945-F957. doi: 10.1152/ajprenal.00353.2016. Epub 2016 Aug 31. Am J Physiol Renal Physiol. 2016. PMID: 27582103
-
Pathophysiological Roles of Ezrin/Radixin/Moesin Proteins.Biol Pharm Bull. 2017;40(4):381-390. doi: 10.1248/bpb.b16-01011. Biol Pharm Bull. 2017. PMID: 28381792 Review.
-
CLIC proteins, ezrin, radixin, moesin and the coupling of membranes to the actin cytoskeleton: a smoking gun?Biochim Biophys Acta. 2014 Feb;1838(2):643-57. doi: 10.1016/j.bbamem.2013.05.025. Epub 2013 Jun 1. Biochim Biophys Acta. 2014. PMID: 23732235 Review.
Cited by
-
3D matrix stiffness modulation unveils cardiac fibroblast phenotypic switching.Sci Rep. 2024 Jul 24;14(1):17015. doi: 10.1038/s41598-024-67646-x. Sci Rep. 2024. PMID: 39043765 Free PMC article.
-
An optogenetic model reveals cell shape regulation through FAK and fascin.J Cell Sci. 2021 Jul 1;134(13):jcs258321. doi: 10.1242/jcs.258321. Epub 2021 Jul 8. J Cell Sci. 2021. PMID: 34114634 Free PMC article.
-
Recent Advances in Engineering the Stem Cell Microniche in 3D.Adv Sci (Weinh). 2018 Jun 13;5(8):1800448. doi: 10.1002/advs.201800448. eCollection 2018 Aug. Adv Sci (Weinh). 2018. PMID: 30128252 Free PMC article. Review.
-
DLITE Uses Cell-Cell Interface Movement to Better Infer Cell-Cell Tensions.Biophys J. 2019 Nov 5;117(9):1714-1727. doi: 10.1016/j.bpj.2019.09.034. Epub 2019 Oct 7. Biophys J. 2019. PMID: 31648791 Free PMC article.
-
Enhanced Dendritic Actin Network Formation in Extended Lamellipodia Drives Proliferation in Growth-Challenged Rac1P29S Melanoma Cells.Dev Cell. 2019 May 6;49(3):444-460.e9. doi: 10.1016/j.devcel.2019.04.007. Dev Cell. 2019. PMID: 31063759 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

