Lipidomics Reveals Cerebrospinal-Fluid Signatures of ALS
- PMID: 29247199
- PMCID: PMC5732162
- DOI: 10.1038/s41598-017-17389-9
Lipidomics Reveals Cerebrospinal-Fluid Signatures of ALS
Abstract
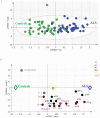
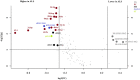
Amyotrophic lateral sclerosis (ALS), the commonest adult-onset motor neuron disorder, is characterized by a survival span of only 2-5 years after onset. Relevant biomarkers or specific metabolic signatures would provide powerful tools for the management of ALS. The main objective of this study was to investigate the cerebrospinal fluid (CSF) lipidomic signature of ALS patients by mass spectrometry to evaluate the diagnostic and predictive values of the profile. We showed that ALS patients (n = 40) displayed a highly significant specific CSF lipidomic signature compared to controls (n = 45). Phosphatidylcholine PC(36:4), higher in ALS patients (p = 0.0003) was the most discriminant molecule, and ceramides and glucosylceramides were also highly relevant. Analysis of targeted lipids in the brain cortex of ALS model mice confirmed the role of some discriminant lipids such as PC. We also obtained good models for predicting the variation of the ALSFRS-r score from the lipidome baseline, with an accuracy of 71% in an independent set of patients. Significant predictions of clinical evolution were found to be correlated to sphingomyelins and triglycerides with long-chain fatty acids. Our study, which shows extensive lipid remodelling in the CSF of ALS patients, provides a new metabolic signature of the disease and its evolution with good predictive performance.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Mitsumoto, H., Brooks, B. R. & Silani, V. Clinical trials in amyotrophic lateral sclerosis: why so many negative trials and how can trials be improved? Lancet Neurol13, 1127–1138, doi:S1474-4422(14)70129-2 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

