Baculovirus-assisted Reovirus Infection in Monolayer and Spheroid Cultures of Glioma cells
- PMID: 29247249
- PMCID: PMC5732240
- DOI: 10.1038/s41598-017-17709-z
Baculovirus-assisted Reovirus Infection in Monolayer and Spheroid Cultures of Glioma cells
Abstract
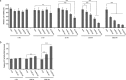
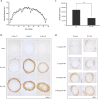
The mammalian orthoreovirus Type 3 Dearing has great potential as oncolytic agent in cancer therapy. One of the bottlenecks that hampers its antitumour efficacy in vivo is the limited tumour-cell infection and intratumoural distribution. This necessitates strategies to improve tumour penetration. In this study we employ the baculovirus Autographa californica multiple nucleopolyhedrovirus as a tool to expand the reovirus' tropism and to improve its spread in three-dimensional tumour-cell spheroids. We generated a recombinant baculovirus expressing the cellular receptor for reovirus, the Junction Adhesion Molecule-A, on its envelope. Combining these Junction Adhesion Molecule-A-expressing baculoviruses with reovirus particles leads to the formation of biviral complexes. Exposure of the reovirus-resistant glioblastoma cell line U-118 MG to the baculovirus-reovirus complexes results in efficient reovirus infection, high reovirus yields, and significant reovirus-induced cytopathic effects. As compared to the reovirus-only incubations, the biviral complexes demonstrated improved penetration and increased cell killing of three-dimensional U-118 MG tumour spheroids. Our data demonstrate that reovirus can be delivered with increased efficiency into two- and three-dimensional tumour-cell cultures via coupling the reovirus particles to baculovirus. The identification of baculovirus' capacity to penetrate into tumour tissue opens novel opportunities to improve cancer therapy by improved delivery of oncolytic viruses into tumours.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

