iTRAQ-Based Quantitative Proteomics Reveals the New Evidence Base for Traumatic Brain Injury Treated with Targeted Temperature Management
- PMID: 29247448
- PMCID: PMC5794703
- DOI: 10.1007/s13311-017-0591-2
iTRAQ-Based Quantitative Proteomics Reveals the New Evidence Base for Traumatic Brain Injury Treated with Targeted Temperature Management
Abstract
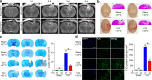
This study aimed to investigate the effects of targeted temperature management (TTM) modulation on traumatic brain injury (TBI) and the involved mechanisms using quantitative proteomics technology. SH-SY5Y and HT-22 cells were subjected to moderate stretch injury using the cell injury controller (CIC), followed by incubation at TTM (mild hypothermia, 32°C), or normothermia (37°C). The real-time morphological changes, cell cycle phase distribution, death, and cell viability were evaluated. Moderate TBI was produced by the controlled cortical impactor (CCI), and the effects of TTM on the neurological damage, neurodegeneration, cerebrovascular histopathology, and behavioral outcome were determined in vivo. Results showed that TTM treatment prevented TBI-induced neuronal necrosis in the brain, achieved a substantial reduction in neuronal death both in vitro and in vivo, reduced cortical lesion volume and neuronal loss, attenuated cerebrovascular histopathological damage, brain edema, and improved behavioral outcome. Using an iTRAQ proteomics approach, proteins that were significantly associated with TTM in experimental TBI were identified. Importantly, changes in four candidate molecules (plasminogen [PLG], antithrombin III [AT III], fibrinogen gamma chain [FGG], transthyretin [TTR]) were verified using TBI rat brain tissues and TBI human cerebrospinal fluid (CSF) samples. This study is one of the first to investigate the neuroprotective effects of TTM on the proteome of human and experimental models of TBI, providing an overall landscape of the TBI brain proteome and a scientific foundation for further assessment of candidate molecules associated with TTM for the promotion of reparative strategies post-TBI.
Keywords: Cerebrospinal fluid; Isobaric tags for relative and absolute quantitation; Proteomics; Targeted temperature management; Traumatic brain injury.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

