Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent
- PMID: 29247502
- PMCID: PMC6486009
- DOI: 10.1002/14651858.CD011300.pub2
Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent
Update in
-
Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent.Cochrane Database Syst Rev. 2021 Dec 6;12(12):CD011300. doi: 10.1002/14651858.CD011300.pub3. Cochrane Database Syst Rev. 2021. PMID: 34870327 Free PMC article.
Abstract
Background: Non-small cell lung cancer (NSCLC) is the most common lung cancer, accounting for approximately 80% to 85% of all cases. For patients with localised NSCLC (stages I to III), it has been speculated that immunotherapy may be helpful for reducing postoperative recurrence rates, or improving the clinical outcomes of current treatment for unresectable tumours. While several new agents have now entered phase III clinical trials, we felt a systematic review was needed to address the question of the effectiveness and safety of immunotherapy in patients with stages I to III NSCLC.
Objectives: To evaluate the effectiveness and safety of immunotherapy (excluding checkpoint inhibitors) in patients with localised NSCLC (stages I to III) who received surgery or radiotherapy with curative intent.
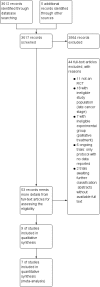
Search methods: We searched the following databases (from inception to 20 January 2017): CENTRAL, MEDLINE, Embase, and CINAHL, and five trial registers. We also manually checked abstracts or reports from relevant conference proceedings and the reference lists of included trials.
Selection criteria: We searched for randomised controlled trials (RCTs) in adults (≥ 18 years) with histologically-confirmed early-stage (stages I to III) NSCLC after surgical resection, and those with unresectable locally advanced stage III NSCLC who had received radiotherapy with curative intent. For patients who had received primary surgical treatment, postoperative radiotherapy or chemoradiotherapy was allowed if it was used for both experimental and control groups.
Data collection and analysis: Two review authors independently selected eligible trials, assessed risk of bias, and extracted data. We used survival analysis to pool time-to-event data, expressing the intervention effect as a hazard ratio (HR). We calculated risk ratios (RR) for dichotomous data, and mean differences for continuous data, with 95% confidence intervals (CI). Due to clinical heterogeneity (immunotherapeutic agents with different underlying mechanisms), we used random-effects models for our meta-analyses.
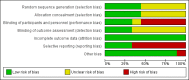
Main results: We identified nine eligible trials that randomised 4940 participants, who had received surgical resection or curative radiotherapy, to either an immunotherapy group or a control group. Included immunological interventions were active immunotherapy (i.e. Bacillus Calmette-Guérin (BCG)), adoptive cell transfer (i.e. transfer factor (TF), tumour-infiltrating lymphocytes (TIL), dendritic cell-cytokine induced killer (DC-CIK), and antigen-specific cancer vaccines (melanoma-associated antigen 3 (MAGE-A3) and L-BLP25). Except for one small trial, which provided insufficient information for risk assessment, we assessed five studies at high risk of bias for at least one of the seven biases studied; we considered the risk of bias in the other three trials to be low. We included data from seven of the nine trials in the meta-analyses (4695 participants). We pooled data from 3693 participants from the three high quality RCTs to evaluate overall survival (OS) and progression-free survival (PFS). We found a small, but not statistically significant, improvement in OS (HR 0.94, 95% CI 0.83 to 1.06; P = 0.35), and PFS (HR 0.93, 95% CI 0.81 to 1.07; P = 0.19; high-quality evidence). The addition of immunotherapy resulted in a small, but not statistically significant, increased risk of having any adverse event (RR 1.15, 95% CI 0.97 to 1.37; P = 0.11, three trials, 3955 evaluated participants, moderate-quality evidence), or severe adverse events (RR 1.10, 95% CI 0.88 to 1.39; four trials, 4362 evaluated participants; low-quality evidence).We analysed data from six studies for one-, two-, and three-year survival rates (4265 participants), and from six studies for five-year survival rates (4234 participants). We observed no clear between-group differences (low-quality evidence for one- and two-year survival rates, and moderate-quality evidence for three- and five-year survival rate).No trial reported the overall response rates; only one trial provided health-related quality of life results.
Authors' conclusions: The current literature does not provide evidence that suggests a survival benefit from adding immunotherapy (excluding checkpoint inhibitors) to conventional curative surgery or radiotherapy, for patients with localised NSCLC (stages I to III). The addition of vaccine-based immunotherapy might increase the risk of adverse events. Several ongoing trials with immune checkpoints inhibitors (PD-1/PD-L1) might bring new insights for role of immunotherapy for patients with stages I to III NSCLC.
Conflict of interest statement
HS declares no conflict of interest.
JZ declares no conflict of interest.
RL declares no conflict of interest.
ET declares no conflict of interest.
RR declares no conflict of interest.
CS declares no conflict of interest.
OT declares no conflict of interest.
Figures











References
References to studies included in this review
-
- Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, et al. Tecemotide (L‐BLP25) versus placebo after chemoradiotherapy for stage III non‐small‐cell lung cancer(START): a randomised, double‐blind, phase 3 trial. Lancet Oncolology 2014;15(1):59‐68. - PubMed
-
- Fujisawa T, Yamaguchi Y. Postoperative immunostimulation after complete resection improves survival of patients with stage I non‐small cell lung carcinoma. Cancer 1996;78(9):1892‐8. - PubMed
-
- Giovanni BR, Paolo Z, Sandro M, Paolo M, Riccardo A, Giovanni F, et al. A randomized trial of adoptive immunotherapy with tumor‐infiltrating lymphocytes and interleukin‐2 versus standard therapy in the postoperative treatment of resected non‐small cell lung carcinoma. Cancer 1996;78(2):244‐51. - PubMed
-
- Macchiarini P, Hardin M, Angeletti CA. Long‐term evaluation of intrapleural Bacillus Calmette‐Guérin with or without adjuvant chemotherapy in completely resected stages II and III non‐small‐cell lung cancer. American Journal of Clinical Oncology 1991;14(4):291‐7. - PubMed
-
- Matthay RA, Mahler DA, Beck GJ, Loke J, Baue AE, Carter DC, et al. Intratumoral Bacillus Calmette‐Guérin immunotherapy prior to surgery for carcinoma of the lung:results of a prospective randomized trial. Cancer Research 1986;46(11):5963‐8. - PubMed
References to studies excluded from this review
-
- Belani CP, Nemunaitis JJ, Chachoua A, Eisenberg PD, Raez LE, Cuevas JD, et al. Phase 2 trial of erlotinib with or without PF‐3512676 (CPG 7909, a Toll‐like receptor 9 agonist) inpatients with advanced recurrent EGFR‐positive non‐small cell lung cancer. Cancer Biology & Therapy 2013 Jul;14(7):557‐63. - PMC - PubMed
-
- García B, Neninger E, Torre A, Leonard I, Martínez R, Viada C, et al. Effective inhibition of the epidermal growth factor/epidermal growth factor receptor binding by anti‐epidermal growth factor antibodies is related to better survival in advanced non‐small‐cell lung cancer patients treated with the epidermal growth factor cancer vaccine. Clinical Cancer Research 2008;14(3):840‐6. - PubMed
-
- Kimura H, Yamaguchi, Y. A phase III randomized study of interleukin‐2 lymphokine‐activated killer cell immunotherapy combined with chemotherapy or radiotherapy after curative or noncurative resection of primary lung carcinoma. Cancer 1997;80(1):42‐9. - PubMed
-
- Kotsakis A. Papadimitraki E, Vetsika EK, Aggouraki D, Dermitzaki EK, Hatzidaki D, et al. A phase II trial evaluating the clinical and immunologic response of HLA‐A2(+) non‐small cell lung cancer patients vaccinated with an hTERT cryptic peptide. Lung Cancer 2014;86(1):59‐66. - PubMed
References to studies awaiting assessment
-
- Macchiarini P, Mussi A, Angeletti CA. Intrapleural BCG in postsurgical stage I non‐small cell lung cancer. Anticancer Research 1989;9(2):391‐3. - PubMed
-
- Schlieben I, Calavrezos A, Koschel G, Seysen U. Adjuvant BCG therapy in radical resection of lung cancer. Praxis und Klinik der Pneumologie 1984;38(3):102‐3. - PubMed
References to ongoing studies
-
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small‐Cell Lung Cancer. The New England Journal of Medicine. 2017 Sep 8;[Epub ahead of print]:doi: 10.1056/NEJMoa1709937. - PubMed
- Haiyan Jiang. A Global Study to Assess the Effects of MEDI4736 Following Concurrent Chemoradiation in Patients With Stage III Unresectable Non‐Small Cell Lung Cancer (PACIFIC). ClinicalTrials.gov.
-
- Canadian Cancer Trials Group. A Phase III Prospective Double Blind Placebo Controlled Randomized Study of Adjuvant MEDI4736 In Completely Resected Non‐Small Cell Lung Cancer. ClinicalTrials.gov.
-
- Merck KGaA. Study of Tecemotide (L‐BLP25) in Participants With Stage III Unresectable Non‐small Cell Lung Cancer (NSCLC) Following Primary Chemoradiotherapy. ClinicalTrials.gov.
-
- Hoffmann‐La Roche. Study to Assess Safety and Efficacy of Atezolizumab (MPDL3280A) Compared to Best Supportive Care Following Chemotherapy in Patients With Lung Cancer. ClinicalTrials.gov.
-
- Sharp M, Corp D. Study of Pembrolizumab (MK‐3475) vs Placebo for Participants With Non‐small Cell Lung Cancer After Resection With or Without Standard Adjuvant Therapy (MK‐3475‐091/KEYNOTE‐091) (PEARLS). ClinicalTrials.gov.
Additional references
-
- Acres B, Limacher JM. MUC1 as a target antigen for cancer immunotherapy. Expert Review of Vaccines 2005;4(4):493‐502. - PubMed
-
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta‐analysis (Statistics in Practice). Chichester: John Wiley & Sons, 2008.
-
- Dasanu CA, Sethi N, Ahmed N. Immune alterations and emerging immunotherapeutic approaches in lung cancer. Expert Opinion on Biological Therapy 2012;12(7):923‐37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

