Rationale and design of the long-Term rIsk, clinical manaGement, and healthcare Resource utilization of stable coronary artery dISease in post-myocardial infarction patients (TIGRIS) study
- PMID: 29247524
- PMCID: PMC6490636
- DOI: 10.1002/clc.22837
Rationale and design of the long-Term rIsk, clinical manaGement, and healthcare Resource utilization of stable coronary artery dISease in post-myocardial infarction patients (TIGRIS) study
Abstract
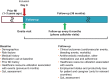
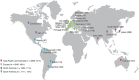
The long-term progression of coronary artery disease as defined by the natural disease course years after a myocardial infarction (MI) is an important but poorly studied area of clinical research. The long-Term rIsk, clinical manaGement, and healthcare Resource utilization of stable coronary artery dISease in post-myocardial infarction patients (TIGRIS) study was designed to address this knowledge gap by evaluating patient management and clinical outcomes following MI in different regions worldwide. TIGRIS (ClinicalTrials.gov Identifier: NCT01866904) is a multicenter, observational, prospective, longitudinal study enrolling patients with history of MI 1 to 3 years previously and high risk of developing atherothrombotic events in a general-practice setting. The primary objective of TIGRIS is to evaluate clinical events (time to first occurrence of any event from the composite cardiovascular endpoint of MI, unstable angina with urgent revascularization, stroke, or death from any cause), and healthcare resource utilization associated with hospitalization for these events (hospitalization duration and procedures) during follow-up. Overall, 9225 patients were enrolled between June 2013 and November 2014 and are being followed in 369 different centers worldwide. This will allow for the description of regional differences in patient characteristics, risk profiles, medical treatment patterns, clinical outcomes, and healthcare resource utilization. Patients will be followed for up to 3 years. Here we report the rationale, design, patient distribution, and selected baseline characteristics of the TIGRIS study. TIGRIS will describe real-world management, quality of life (self-reported health), and healthcare resource utilization for patients with stable coronary artery disease ≥1 year post-MI.
Keywords: Coronary artery disease; Healthcare resource utilization; Observational; Trial design; myocardial infarction < Ischemic heart disease.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
Dirk Westermann reports speaker/consulting honoraria and/or research grant support from AstraZeneca, Bayer, Berlin‐Chemie, Biotronik, and Novartis. Shaun G. Goodman reports speaker/consulting honoraria and/or research grant support from Amgen Inc., Amilyn, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Ferring Pharmaceuticals, GlaxoSmithKline, Matrizyme, Merck, Novartis, Pfizer, Regeneron, Revalesio, Sanofi, Servier, and Tenax Pharmaceuticals. José C. Nicolau reports speaker/consulting honoraria and/or research grant support from Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, GlaxoSmithKline, Merck, Novartis, Pfizer, and Sanofi. Gema Requena and Andrew Maguire are employed by Oxon Epidemiology Ltd. (which has received funding from AstraZeneca). Christopher B. Granger reports consulting honoraria and/or research grant support from Armetheon, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Eli Lilly, Gilead, GlaxoSmithKline, Hoffmann‐La Roche, Janssen Pharmaceuticals, Medtronic, Pfizer, Salix Pharmaceuticals, Sanofi, Takeda, and The Medicines Company. Stuart Pocock reports statistical consulting honoraria from AstraZeneca. Stefan Blankenberg reports speaker/consulting honoraria and/or research grant support from Abbott, Abbott Diagnostics, AstraZeneca, Bayer, Boehringer Ingelheim, Medtronic, Novartis, Pfizer, Roche, Siemens, Siemens Diagnostics, and Thermo Fisher Scientific. Tabassome Simon reports speaker/consulting honoraria and/or research grant support from Astellas, Amgen Inc., AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Pfizer, and Sanofi. Satoshi Yasuda reports speaker/consulting honoraria and/or research grant support from Takeda, Daiichi‐Sankyo, AstraZeneca, Boehringer Ingelheim, and Bristol‐Myers Squibb. Ana Maria Vega is employed by AstraZeneca. David Brieger reports speaker/consulting honoraria and/or research grant support from Amgen Inc., AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Merck, and Sanofi. The authors declare no other potential conflicts of interest.
Figures
References
-
- Wallentin L, Becker RC, Budaj A, et al; PLATO Investigators . Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. - PubMed
-
- Mariani M, Mariani G, De Servi S. Efficacy and safety of prasugrel compared with clopidogrel in patients with acute coronary syndromes: results of TRITON‐TIMI 38 trials. Exp Rev Cardiovasc Ther. 2009;7:17–23. - PubMed
-
- Sibbing D, Aradi D, Jacobshagen C, et al; TROPICAL‐ACS Investigators . Guided de‐escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL‐ACS): a randomised, open‐label, multicentre trial. Lancet. 2017;390:1747–57. - PubMed
-
- Udell JA, Bonaca MP, Collet JP, et al. Long‐term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta‐analysis of randomized trials. Eur Heart J. 2016;37:390–399. - PubMed
-
- Simms AD, Weston CF, West RM, et al. Mortality and missed opportunities along the pathway of care for ST‐elevation myocardial infarction: a national cohort study. Eur Heart J Acute Cardiovasc Care. 2015;4:241–253. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

