A randomized controlled trial of screening and brief interventions for substance misuse in reproductive health
- PMID: 29247636
- PMCID: PMC6896206
- DOI: 10.1016/j.ajog.2017.12.005
A randomized controlled trial of screening and brief interventions for substance misuse in reproductive health
Abstract
Background: Screening, brief intervention, and referral to treatment may reduce substance misuse but has received minimal study among women who are treated in reproductive health settings.
Objective: The purpose of this study was to determine whether "screening, brief intervention and referral to treatment" that is delivered either electronically or by clinician are more effective than enhanced usual care in decreasing days of primary substance use.
Study design: Women from 2 reproductive centers who smoked cigarettes or misused alcohol, illicit drugs, or prescription medication were allocated randomly to "screening, brief intervention and referral to treatment" delivered electronically or by clinician or to enhanced usual care. Assessments were completed at baseline and at 1-, 3-, and 6-months after a baseline has been established. Coprimary outcomes were days/months of primary substance use and postintervention treatment use. A sample size of 660 women was planned; randomization was stratified by primary substance use and pregnancy status. "Screening, brief intervention and referral to treatment" groups were compared with enhanced usual care groups with the use of generalized estimation equations, and effect sizes were calculated with the use of Cohen's d.
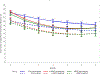
Results: Between September 2011 and January 2015, women were assigned randomly to a group: 143 women (16.8% pregnant) in the electronic-delivered "screening, brief intervention and referral to treatment" group, 145 women (18.6% pregnant) in the clinician-delivered "screening, brief intervention and referral to treatment" group, and 151 women (19.2% pregnant) in the enhanced usual care group; the retention was >84%. Based on the generalized estimating equations model, predicted mean days per month of use at baseline for primary substance were 23.9 days (95% confidence interval, 22.4-25.5) for the electronic-delivered group, 22.8 days (95% confidence interval, 21.4-24.3) for the clinician-delivered group, and 23.5 days (95% confidence interval, 22.2, 24.9) for enhanced usual care, which respectively declined to 20.5 days (95% confidence interval, 19.0-22.2), 19.8 days (95% confidence interval,18.5-21.3), and 21.9 days (95% confidence interval, 20.7-23.1) at 1 month; 16.9 days (95% confidence interval, 15.0-19.0), 16.6 days (95% confidence interval, 14.8-18.6), and 19.5 days (95% confidence interval, 18.1-21.1) at 3 months; and 16.3 days (95% confidence interval, 14.3-18.7), 16.3 days (95% confidence interval, 14.4-18.5), and 17.9 days (95% confidence interval, 16.1-19.9) at 6 months. Estimated declines were greater in the electronic-delivered group (β [standard error]=-0.090[0.034]; P=.008; Cohen's d, 0.19 at 1 month, 0.30 at 3 months, and 0.17 at 6 months) and the clinician-delivered group (β [standard error]=-0.078[0.037]; P=.038; Cohen's d, 0.17 at 1 month, 0.22 at 3 months, and 0.06 at 6 months) compared with enhanced usual care. Treatment use did not differ between groups.
Conclusion: "Screening, brief intervention and referral to treatment" significantly decreased days of primary substance use among women in reproductive healthcare centers; neither resulted in more treatment use than enhanced usual care.
Keywords: brief intervention; motivational interviewing; primary care; reproductive health; screening; substance use; treatment use.
Copyright © 2017. Published by Elsevier Inc.
Conflict of interest statement
Figures
References
-
- SAMHSA. Results from the 2013 National Survey on Drug Use and Health: summary of National Findings. In: Administration SAaMHS, ed. Rockville, MD, 2014.
-
- BERTHOLET N, DAEPPEN JB, WIETLISBACH V, FLEMING M, BURNAND B. Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis. Archives of Internal Medicine 2005;165:986–95. - PubMed
-
- WHITLOCK EP, POLEN MR, GREEN CA, ORLEANS T, KLEIN J, FORCE USPST. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2004;140:557–68. - PubMed
-
- STEAD LF, BERGSON G, LANCASTER T. Physician advice for smoking cessation.[update of Cochrane Database Syst Rev. 2004;(4):CD000165; PMID: 15494989]. Cochrane Database of Systematic Reviews 2008:CD000165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

