Effects of a novel microtubule-depolymerizer on pro-inflammatory signaling in RAW264.7 macrophages
- PMID: 29247640
- PMCID: PMC5766364
- DOI: 10.1016/j.cbi.2017.12.019
Effects of a novel microtubule-depolymerizer on pro-inflammatory signaling in RAW264.7 macrophages
Abstract
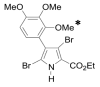
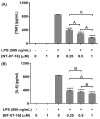
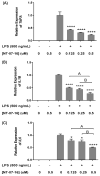
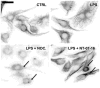
The Nuclear Factor-kappa B (NF-κB) pathway is vital for immune system regulation and pro-inflammatory signaling. Many inflammatory disorders and diseases, including cancer, are linked to dysregulation of NF-κB signaling. When macrophages recognize the presence of a pathogen, the signaling pathway is activated, resulting in the nuclear translocation of the transcription factor, NF-κB, to turn on pro-inflammatory genes. Here, we demonstrate the effects of a novel microtubule depolymerizer, NT-07-16, a polysubstituted pyrrole compound, on this process. Treatment with NT-07-16 decreased the production of pro-inflammatory cytokines in RAW264.7 mouse macrophages. It appears that the reduction in pro-inflammatory mediators produced by the macrophages after exposure to NT-07-16 may be due to activities upstream of the translocation of NF-κB into the nucleus. NF-κB translocation occurs after its inhibitory protein, IκB-α is phosphorylated which signals for its degradation releasing NF-κB so it is free to move into the nucleus. Previous studies from other laboratories indicate that these processes are associated with the microtubule network. Our results show that exposure to the microtubule-depolymerizer, NT-07-16 reduces the phosphorylation of IκB-α and also decreases the association of NF-κB with tubulin which may affect the ability of NF-κB to translocate into the nucleus. Therefore, the anti-inflammatory activity of NT-07-16 may be explained, at least in part, by alterations in these steps in the NF-κB signaling pathway leading to less NF-κB entering the nucleus and reducing the production of pro-inflammatory mediators by the activated macrophages.
Keywords: Inflammation; Macrophage; Microtubule; NF-κB; NT-07-16 pyrrole compound; Signaling.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures


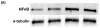
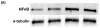

References
-
- Antonelli A, Kushner I. It’s time to redefine inflammation. FASEB J. 2017;31:1787–1791. - PubMed
-
- Kondylis V, Kumari S, Vlantis K, Pasparakis M. The interplay of IKK, NF- B and RIPK1 signaling in the regulation of cell death, tissue homeostasis and inflammation. Immunol Rev. 2017;277:113–127. - PubMed
-
- Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. - PubMed
-
- Komai K, Shichita T, Ito M, Kanamori M, Chikuma S. Role of scavenger receptors as damage-associated molecular pattern receptors in Toll-like receptor activation. Int Immunol. 2017;29:59–70. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

