Adrenergic and glucocorticoid receptor antagonists reduce ozone-induced lung injury and inflammation
- PMID: 29247675
- PMCID: PMC7110430
- DOI: 10.1016/j.taap.2017.12.006
Adrenergic and glucocorticoid receptor antagonists reduce ozone-induced lung injury and inflammation
Abstract
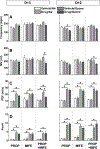
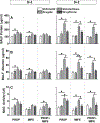
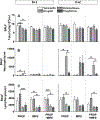
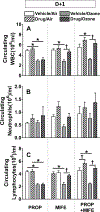
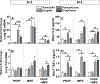
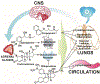
Recent studies showed that the circulating stress hormones, epinephrine and corticosterone/cortisol, are involved in mediating ozone-induced pulmonary effects through the activation of the sympathetic-adrenal-medullary (SAM) and hypothalamus-pituitary-adrenal (HPA) axes. Hence, we examined the role of adrenergic and glucocorticoid receptor inhibition in ozone-induced pulmonary injury and inflammation. Male 12-week old Wistar-Kyoto rats were pretreated daily for 7days with propranolol (PROP; a non-selective β adrenergic receptor [AR] antagonist, 10mg/kg, i.p.), mifepristone (MIFE; a glucocorticoid receptor [GR] antagonist, 30mg/kg, s.c.), both drugs (PROP+MIFE), or respective vehicles, and then exposed to air or ozone (0.8ppm), 4h/d for 1 or 2 consecutive days while continuing drug treatment. Ozone exposure alone led to increased peak expiratory flow rates and enhanced pause (Penh); with greater increases by day 2. Receptors blockade minimally affected ventilation in either air- or ozone-exposed rats. Ozone exposure alone was also associated with marked increases in pulmonary vascular leakage, macrophage activation, neutrophilic inflammation and lymphopenia. Notably, PROP, MIFE and PROP+MIFE pretreatments significantly reduced ozone-induced pulmonary vascular leakage; whereas PROP or PROP+MIFE reduced neutrophilic inflammation. PROP also reduced ozone-induced increases in bronchoalveolar lavage fluid (BALF) IL-6 and TNF-α proteins and/or lung Il6 and Tnfα mRNA. MIFE and PROP+MIFE pretreatments reduced ozone-induced increases in BALF N-acetyl glucosaminidase activity, and lymphopenia. We conclude that stress hormones released after ozone exposure modulate pulmonary injury and inflammatory effects through AR and GR in a receptor-specific manner. Individuals with pulmonary diseases receiving AR and GR-related therapy might experience changed sensitivity to air pollution.
Keywords: Adrenergic receptor antagonist; Glucocorticoid receptor antagonist; Lung inflammation; Lung injury; Ozone; Stress response.
Published by Elsevier Inc.
Figures


Similar articles
-
Independent roles of beta-adrenergic and glucocorticoid receptors in systemic and pulmonary effects of ozone.Inhal Toxicol. 2020 Mar;32(4):155-169. doi: 10.1080/08958378.2020.1759736. Epub 2020 May 4. Inhal Toxicol. 2020. PMID: 32366144 Free PMC article.
-
Beta-2 Adrenergic and Glucocorticoid Receptor Agonists Modulate Ozone-Induced Pulmonary Protein Leakage and Inflammation in Healthy and Adrenalectomized Rats.Toxicol Sci. 2018 Dec 1;166(2):288-305. doi: 10.1093/toxsci/kfy198. Toxicol Sci. 2018. PMID: 30379318 Free PMC article.
-
Adrenergic receptor subtypes differentially influence acrolein-induced ventilatory, vascular leakage, and inflammatory responses.Toxicol Appl Pharmacol. 2025 May;498:117303. doi: 10.1016/j.taap.2025.117303. Epub 2025 Mar 16. Toxicol Appl Pharmacol. 2025. PMID: 40101861
-
Neuroendocrine Regulation of Air Pollution Health Effects: Emerging Insights.Toxicol Sci. 2018 Jul 1;164(1):9-20. doi: 10.1093/toxsci/kfy129. Toxicol Sci. 2018. PMID: 29846720 Free PMC article. Review.
-
Adrenergic and Glucocorticoid Receptors in the Pulmonary Health Effects of Air Pollution.Toxics. 2021 Jun 4;9(6):132. doi: 10.3390/toxics9060132. Toxics. 2021. PMID: 34200050 Free PMC article. Review.
Cited by
-
Sympathetic System in Wound Healing: Multistage Control in Normal and Diabetic Skin.Int J Mol Sci. 2023 Jan 20;24(3):2045. doi: 10.3390/ijms24032045. Int J Mol Sci. 2023. PMID: 36768369 Free PMC article. Review.
-
Characterization of low-dose ozone-induced murine acute lung injury.Physiol Rep. 2020 Jun;8(11):e14463. doi: 10.14814/phy2.14463. Physiol Rep. 2020. PMID: 32524776 Free PMC article.
-
Differential transcriptomic alterations in nasal versus lung tissue of acrolein-exposed rats.Front Toxicol. 2023 Nov 27;5:1280230. doi: 10.3389/ftox.2023.1280230. eCollection 2023. Front Toxicol. 2023. PMID: 38090360 Free PMC article.
-
Transcriptional Effects of Ozone and Impact on Airway Inflammation.Front Immunol. 2019 Jul 10;10:1610. doi: 10.3389/fimmu.2019.01610. eCollection 2019. Front Immunol. 2019. PMID: 31354743 Free PMC article. Review.
-
Individual and joint effects of prenatal PM2.5 and maternal stress on child temperament.Environ Res. 2024 May 15;249:118432. doi: 10.1016/j.envres.2024.118432. Epub 2024 Feb 12. Environ Res. 2024. PMID: 38354885 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

