PD-1 Modulates Radiation-Induced Cardiac Toxicity through Cytotoxic T Lymphocytes
- PMID: 29247829
- PMCID: PMC9335897
- DOI: 10.1016/j.jtho.2017.12.002
PD-1 Modulates Radiation-Induced Cardiac Toxicity through Cytotoxic T Lymphocytes
Abstract
Introduction: Combined immune checkpoint blockade has led to rare autoimmune complications, such as fatal myocarditis. Recent approvals of several anti-programmed death 1 (anti-PD-1) drugs for lung cancer treatment prompted ongoing clinical trials that directly combine PD-1 inhibitors with thoracic radiotherapy for locally advanced lung cancer. Overlapping toxicities from either modality have the potential to increase the risk for radiation-induced cardiotoxicity (RICT), which is well documented among patients with Hodgkin's disease and breast cancer.
Methods: To investigate cardiotoxicity without the compounding pulmonary toxicity from thoracic radiotherapy, we developed a technique to deliver cardiac irradiation (CIR) in a mouse model concurrently with PD-1 blockade to determine the presence of cardiac toxicity by using physiological testing and mortality as end points along with histological analysis.
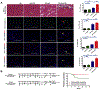
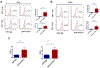
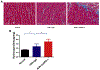
Results: We observed an acute mortality of 30% within 2 weeks after CIR plus anti-PD-1 antibody compared with 0% from CIR plus immunoglobulin G (p = 0.023). Physiological testing demonstrated a reduced left ventricular ejection fraction (p < 0.01) by echocardiogram. Tissue analyses revealed increased immune cell infiltrates within cardiac tissue. Depletion of CD8-positive lymphocytes with anti-CD8 antibody reversed the acute mortality, suggesting that the toxicity is CD8-positive cell-mediated. To validate these findings using a clinically relevant fractionated radiotherapy regimen, we repeated the study by delivering five daily fractions of 6 Gy. Similar mortality, cardiac dysfunction, and histological changes were observed in mice receiving fractionated radiotherapy with concurrent anti-PD-1 therapy.
Conclusions: This study provides strong preclinical evidence that radiation-induced cardiotoxicity is modulated by the PD-1 axis and that PD-1 blockade should be administered with careful radiotherapy planning with an effort of reducing cardiac dose.
Keywords: CD8; Immunotherapy; PD-1; Radiation-induced cardiac toxicity; Radiotherapy; Toxicity.
Copyright © 2017 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure: The authors declare no conflict of interest.
Figures



Comment in
-
PD-(L)1 Inhibition and Cardiac Damage: A Relevant Toxicity?J Thorac Oncol. 2018 Apr;13(4):478-479. doi: 10.1016/j.jtho.2018.02.008. J Thorac Oncol. 2018. PMID: 29576288 No abstract available.
References
-
- Kaplan BM, Miller AJ, Bharati S, Lev M, Martin Grais I. Complete AV block following mediastinal radiation therapy: electrocardiographic and pathologic correlation and review of the world literature. J Interv Card Electrophysiol. 1997;1:175–188. - PubMed
-
- Aleman BMP, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. - PubMed
-
- Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–3148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

