Knockout of l-Histidine Decarboxylase Prevents Cholangiocyte Damage and Hepatic Fibrosis in Mice Subjected to High-Fat Diet Feeding via Disrupted Histamine/Leptin Signaling
- PMID: 29248461
- PMCID: PMC5840487
- DOI: 10.1016/j.ajpath.2017.11.016
Knockout of l-Histidine Decarboxylase Prevents Cholangiocyte Damage and Hepatic Fibrosis in Mice Subjected to High-Fat Diet Feeding via Disrupted Histamine/Leptin Signaling
Abstract
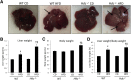
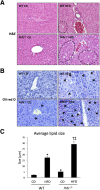
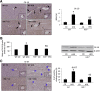
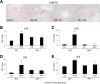
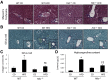
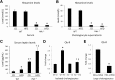
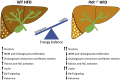
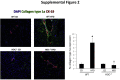
Feeding a high-fat diet (HFD) coupled with sugar, mimicking a Western diet, causes fatty liver disease in mice. Histamine induces biliary proliferation and fibrosis and regulates leptin signaling. Wild-type (WT) and l-histidine decarboxylase (Hdc-/-) mice were fed a control diet or an HFD coupled with a high fructose corn syrup equivalent. Hematoxylin and eosin and Oil Red O staining were performed to determine steatosis. Biliary mass and cholangiocyte proliferation were evaluated by immunohistochemistry. Senescence and fibrosis were measured by quantitative PCR and immunohistochemistry. Hepatic stellate cell activation was detected by immunofluorescence. Histamine and leptin levels were measured by enzyme immunoassay. Leptin receptor (Ob-R) was evaluated by quantitative PCR. The HDC/histamine/histamine receptor axis, ductular reaction, and biliary senescence were evaluated in patients with nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, or end-stage liver disease. Hdc-/- HFD mice had increased steatosis compared with WT HFD mice. WT HFD mice had increased biliary mass, biliary proliferation, senescence, fibrosis, and hepatic stellate cell activation, which were reduced in Hdc-/- HFD mice. In Hdc-/- HFD mice, serum leptin levels increased, whereas biliary Ob-R expression decreased. Nonalcoholic steatohepatitis patients had increased HDC/histamine/histamine receptor signaling. Hdc-/- HFD mice are susceptible to obesity via dysregulated leptin/Ob-R signaling, whereas the lack of HDC protects from HFD-induced fibrosis and cholangiocyte damage. HDC/histamine/leptin signaling may be important in managing obesity-induced biliary damage.
Copyright © 2018 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Fitzgerald K.R. Review of article: prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010 by Katherine M. Flegal, PhD; Margaret D. Carroll, MSPH; Brian K. Kit, MD; Cynthia L. Ogden, PhD (JAMA 2012;307:491-7) J Vasc Nurs. 2013;31:131–132. - PubMed
-
- Brunt E.M. Pathology of nonalcoholic steatohepatitis. Hepatol Res. 2005;33:68–71. - PubMed
-
- Bugianesi E., Marietti M. Non-alcoholic fatty liver disease (NAFLD)Recenti Prog Med. 2016;107:360–368. Italian. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

