Extracellular matrix directs phenotypic heterogeneity of activated fibroblasts
- PMID: 29248556
- PMCID: PMC5910258
- DOI: 10.1016/j.matbio.2017.12.003
Extracellular matrix directs phenotypic heterogeneity of activated fibroblasts
Abstract
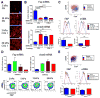
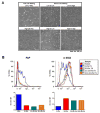
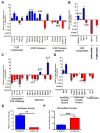
Activated fibroblasts are key players in the injury response, tumorigenesis, fibrosis, and inflammation. Dichotomous outcomes in response to varied stroma-targeted therapies in cancer emphasize the need to disentangle the roles of heterogeneous fibroblast subsets in physiological and pathophysiological settings. In wound healing, fibrosis, and myriad tumor types, fibroblast activation protein (FAP) and alpha-smooth muscle actin (αSMA) identify distinct, yet overlapping, activated fibroblast subsets. Prior studies established that FAPHi reactive fibroblasts and αSMAHi myofibroblasts can exert opposing influences in tumorigenesis. However, the factors that drive this phenotypic heterogeneity and the unique functional roles of these subsets have not been defined. We demonstrate that a convergence of ECM composition, elasticity, and transforming growth factor beta (TGF-β) signaling governs activated fibroblast phenotypic heterogeneity. Furthermore, FAPHi reactive fibroblasts and αSMAHi myofibroblasts exhibited distinct gene expression signatures and functionality in vitro, illuminating potentially unique roles of activated fibroblast subsets in tissue remodeling. These insights into activated fibroblast heterogeneity will inform the rational design of stroma-targeted therapies for cancer and fibrosis.
Keywords: Activated fibroblasts; Alpha-smooth muscle actin; Fibroblast activation protein; Fibroblast heterogeneity.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures



References
-
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. http://dx.doi.org/10.1038/nrm809. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

