Human c-SRC kinase (CSK) overexpression makes T cells dummy
- PMID: 29248956
- PMCID: PMC11028372
- DOI: 10.1007/s00262-017-2105-9
Human c-SRC kinase (CSK) overexpression makes T cells dummy
Abstract
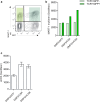
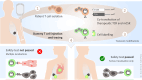
Adoptive cell therapy with T-cell receptor (TCR)-engineered T cells represents a powerful method to redirect the immune system against tumours. However, although TCR recognition is restricted to a specific peptide-MHC (pMHC) complex, increasing numbers of reports have shown cross-reactivity and off-target effects with severe consequences for the patients. This demands further development of strategies to validate TCR safety prior to clinical use. We reasoned that the desired TCR signalling depends on correct pMHC recognition on the outside and a restricted clustering on the inside of the cell. Since the majority of the adverse events are due to TCR recognition of the wrong target, we tested if blocking the signalling would affect the binding. By over-expressing the c-SRC kinase (CSK), a negative regulator of LCK, in redirected T cells, we showed that peripheral blood T cells inhibited anti-CD3/anti-CD28-induced phosphorylation of ERK, whereas TCR proximal signalling was not affected. Similarly, overexpression of CSK together with a therapeutic TCR prevented pMHC-induced ERK phosphorylation. Downstream effector functions were also almost completely blocked, including pMHC-induced IL-2 release, degranulation and, most importantly, target cell killing. The lack of effector functions contrasted with the unaffected TCR expression, pMHC recognition, and membrane exchange activity (trogocytosis). Therefore, co-expression of CSK with a therapeutic TCR did not compromise target recognition and binding, but rendered T cells incapable of executing their effector functions. Consequently, we named these redirected T cells "dummy T cells" and propose to use them for safety validation of new TCRs prior to therapy.
Keywords: CSK; Immunotherapy; T-cell receptor; TCR; TCR signalling.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

