Metronomic capecitabine as second-line treatment for hepatocellular carcinoma after sorafenib discontinuation
- PMID: 29249005
- PMCID: PMC11813436
- DOI: 10.1007/s00432-017-2556-6
Metronomic capecitabine as second-line treatment for hepatocellular carcinoma after sorafenib discontinuation
Abstract
Purpose: Metronomic capecitabine (MC) is a well-tolerated systemic treatment showing promising results in one retrospective study, as second-line therapy after sorafenib failure, in patients with hepatocellular carcinoma (HCC).
Methods: 117 patients undergoing MC were compared to 112 patients, eligible for this treatment, but undergoing best supportive care (BSC) after sorafenib discontinuation for toxicity or HCC progression. The two groups were compared for demographic and clinical features. A multivariate regression analysis was conducted to detect independent prognostic factors. To balance confounding factors between the two groups, a propensity score model based on independent prognosticators (performance status, neoplastic thrombosis, causes of sorafenib discontinuation and pre-sorafenib treatment) was performed.
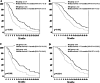
Results: Patients undergoing MC showed better performance status, lower tumor burden, lower prevalence of portal vein thrombosis, and better cancer stage. Median (95% CI) post-sorafenib survival (PSS) was longer in MC than in BSC patients [9.5 (7.5-11.6) vs 5.0 (4.2-5.7) months (p < 0.001)]. Neoplastic thrombosis, cause of sorafenib discontinuation, pre-sorafenib treatment and MC were independent prognosticators. The benefit of capecitabine was confirmed in patients after matching with propensity score [PSS: 9.9 (6.8-12.9) vs. 5.8 (4.8-6.8) months, (p = 0.001)]. MC lowered the mortality risk by about 40%. MC achieved better results in patients who stopped sorafenib for adverse events than in those who progressed during it [PSS: 17.3 (10.5-24.1) vs. 7.8 (5.2-10.1) months, (p = 0.035)]. Treatment toxicity was low and easily manageable with dose modulation.
Conclusions: MC may be an efficient and safe second-line systemic therapy for HCC patients who discontinued sorafenib for toxicity or tumor progression.
Keywords: Hepatocellular carcinoma; Metronomic capecitabine; Second-line chemotherapy; Survival; Toxicity.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Abdel-Rahman O, Abdel-Wahab M, Shaker M, Abdel-Wahab S, Elbassiony M, Ellithy M (2013) Sorafenib versus capecitabine in the management of advanced hepatocellular carcinoma. Med Oncol 30:655 - PubMed
-
- Brandi G, de Rosa F, Bolondi L, Agostini V, Di Girolamo S, Nobili E, Biasco G (2010) Durable complete response of hepatocellular carcinoma after metronomic capecitabine. Tumori 96:1028–1030 - PubMed
-
- Brandi G, de Rosa F, Agostini V, di Girolamo S, Andreone P, Bolondi L, Serra C, Sama C, Golfieri R, Gramenzi A, Cucchetti A, Pinna AD, Trevisani F, Biasco G, Italian Liver Cancer (ITA.LI.CA) Group (2013) Metronomic capecitabine in advanced hepatocellular carcinoma patients: a phase II study. Oncologist 18:1256–1257 - PMC - PubMed
-
- Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J, EASL Panel of Experts on HCC (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35:421–430 - PubMed
-
- Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases (2005) Management of hepatocellular carcinoma. Hepatology 42:1208–1236 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

