T Cells Primed by Live Mycobacteria Versus a Tuberculosis Subunit Vaccine Exhibit Distinct Functional Properties
- PMID: 29249639
- PMCID: PMC5828549
- DOI: 10.1016/j.ebiom.2017.12.004
T Cells Primed by Live Mycobacteria Versus a Tuberculosis Subunit Vaccine Exhibit Distinct Functional Properties
Abstract
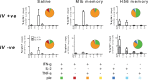
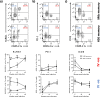
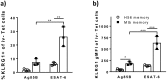
Despite inducing strong T cell responses, Mycobacterium tuberculosis (Mtb) infection fails to elicit protective immune memory. As such latently infected or successfully treated Tuberculosis (TB) patients are not protected against recurrent disease. Here, using a mouse model of aerosol Mtb infection, we show that memory immunity to H56/CAF01 subunit vaccination conferred sustained protection in contrast to the transient natural immunity conferred by Mtb infection. Loss of protection to re-infection in natural Mtb memory was temporally linked to an accelerated differentiation of ESAT-6- and to a lesser extent, Ag85B-specific CD4 T cells in both the lung parenchyma and vasculature. This phenotype was characterized by high KLRG1 expression and low, dual production of IFN-γ and TNF. In contrast, H56/CAF01 vaccination elicited cells that expressed low levels of KLRG1 with copious expression of IL-2 and IL-17A. Co-adoptive transfer studies revealed that H56/CAF01 induced memory CD4 T cells efficiently homed into the lung parenchyma of mice chronically infected with Mtb. In comparison, natural Mtb infection- and BCG vaccine-induced memory CD4 T cells exhibited a poor ability to home into the lung parenchyma. These studies suggest that impaired lung migratory capacity is an inherent trait of the terminally differentiated memory responses primed by mycobacteria/mycobacterial vectors.
Keywords: Lung homing; M. tuberculosis; Natural immunity; T cell priming & differentiation; Vaccination.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Aagaard C., Hoang T., Dietrich J., Cardona P.J., Izzo A., Dolganov G., Schoolnik G.K., Cassidy J.P., Billeskov R., Andersen P. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat. Med. 2011;17:189–U224. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

