β2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer
- PMID: 29249692
- PMCID: PMC5760435
- DOI: 10.1016/j.ccell.2017.11.007
β2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer
Erratum in
-
β2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer.Cancer Cell. 2018 Nov 12;34(5):863-867. doi: 10.1016/j.ccell.2018.10.010. Cancer Cell. 2018. PMID: 30423300 Free PMC article. No abstract available.
Abstract
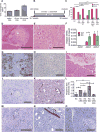
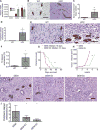
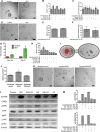
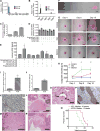
Catecholamines stimulate epithelial proliferation, but the role of sympathetic nerve signaling in pancreatic ductal adenocarcinoma (PDAC) is poorly understood. Catecholamines promoted ADRB2-dependent PDAC development, nerve growth factor (NGF) secretion, and pancreatic nerve density. Pancreatic Ngf overexpression accelerated tumor development in LSL-Kras+/G12D;Pdx1-Cre (KC) mice. ADRB2 blockade together with gemcitabine reduced NGF expression and nerve density, and increased survival of LSL-Kras+/G12D;LSL-Trp53+/R172H;Pdx1-Cre (KPC) mice. Therapy with a Trk inhibitor together with gemcitabine also increased survival of KPC mice. Analysis of PDAC patient cohorts revealed a correlation between brain-derived neurotrophic factor (BDNF) expression, nerve density, and increased survival of patients on nonselective β-blockers. These findings suggest that catecholamines drive a feedforward loop, whereby upregulation of neurotrophins increases sympathetic innervation and local norepinephrine accumulation.
Keywords: NGF-BDNF; TRK; adrenergic signaling; neurotrophins; pancreatic cancer; stress; β-blockers.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

Comment in
-
An Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer.Cancer Discov. 2018 Feb;8(2):138. doi: 10.1158/2159-8290.CD-RW2018-003. Epub 2018 Jan 5. Cancer Discov. 2018. PMID: 29305494
References
-
- Apte MV, Wilson JS. Dangerous liaisons: pancreatic stellate cells and pancreatic cancer cells. J Gastroenterol Hepatol. 2012;27(Suppl 2):69–74. - PubMed
-
- Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. Journal of Clinical Oncology. 2011;29:2635–2644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

