Uncovering the mechanisms of Acinetobacter baumannii virulence
- PMID: 29249812
- PMCID: PMC6571207
- DOI: 10.1038/nrmicro.2017.148
Uncovering the mechanisms of Acinetobacter baumannii virulence
Abstract
Acinetobacter baumannii is a nosocomial pathogen that causes ventilator-associated as well as bloodstream infections in critically ill patients, and the spread of multidrug-resistant Acinetobacter strains is cause for concern. Much of the success of A. baumannii can be directly attributed to its plastic genome, which rapidly mutates when faced with adversity and stress. However, fundamental virulence mechanisms beyond canonical drug resistance were recently uncovered that enable A. baumannii and, to a limited extent, other medically relevant Acinetobacter species to successfully thrive in the health-care environment. In this Review, we explore the molecular features that promote environmental persistence, including desiccation resistance, biofilm formation and motility, and we discuss the most recently identified virulence factors, such as secretion systems, surface glycoconjugates and micronutrient acquisition systems that collectively enable these pathogens to successfully infect their hosts.
Figures
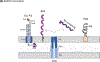
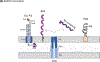
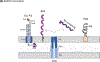
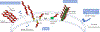

References
-
- (CDC), C. f. D. C. a. P. Antibiotic Resistance Threats in the United States, 2013, <https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf> (2013).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

