Halide Control of N,N- Coordination versus N,C-Cyclometalation and Stereospecific Phenyl Ring Deuteration of Osmium(II) p-Cymene Phenylazobenzothiazole Complexes
- PMID: 29249848
- PMCID: PMC5726741
- DOI: 10.1021/acs.organomet.7b00501
Halide Control of N,N- Coordination versus N,C-Cyclometalation and Stereospecific Phenyl Ring Deuteration of Osmium(II) p-Cymene Phenylazobenzothiazole Complexes
Abstract
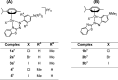
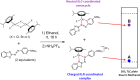
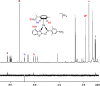
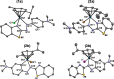
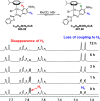
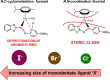
We report the synthesis of halido Os(II) p-cymene complexes bearing bidentate chelating phenylazobenzothiazole (AZBTZ) ligands. Unlike the analogous phenylazopyridine (AZPY) complexes, AZBTZ-NMe2 is capable of both N,N-coordination to Os(II) and cyclometalation to form N,C-coordinated species. N,C-Coordination occurs via an azo nitrogen and an ortho carbon on the aniline ring, as identified by 1H NMR and X-ray crystallography of [Os(p-cym)(N,N-AZBTZ-NMe2)Cl]PF6 (1a), [Os(p-cym)(N,N-AZBTZ-NMe2)Br]PF6 (2a), [Os(p-cym)(N,C-AZBTZ-NMe2)Br] (2b), and [Os(p-cym)(N,C-AZBTZ-NMe2)I] (3b). The N,C-coordinated species is more stable and is not readily converted to the N,N-coordinated complex. Analysis of the crystal structures suggests that their formation is influenced by steric interactions between the p-cym and AZBTZ-NMe2 ligands: in particular, larger monodentate halide ligands favor N,C-coordination. The complexes [Os(p-cym)(N,N-Me2-AZBTZ-NH2)Cl]PF6 (4) and [Os(p-cym)(N,N-Me2-AZBTZ-NH2)I]PF6 (5) were synthesized with methyl groups blocking the ortho positions on the aniline ring, forcing an N,N-coordination geometry. 1H NMR NOE experiments confirmed hindered rotation of the arene ligand and steric crowding around the metal center. Complex 2b exhibited unexpected behavior under acidic conditions, involving regiospecific deuteration of the aniline ring at the meta position, as observed by 1H NMR and high-resolution ESI-MS. Deuterium exchange occurs only under acidic conditions, suggesting an associative mechanism. The calculated partial charges on 2b show that the meta carbon is significantly more negatively charged, which may account for the regiospecificity of deuterium exchange.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
