Peli1 Contributions in Microglial Activation, Neuroinflammatory Responses and Neurological Deficits Following Experimental Subarachnoid Hemorrhage
- PMID: 29249938
- PMCID: PMC5714869
- DOI: 10.3389/fnmol.2017.00398
Peli1 Contributions in Microglial Activation, Neuroinflammatory Responses and Neurological Deficits Following Experimental Subarachnoid Hemorrhage
Abstract
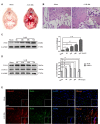
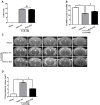
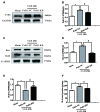
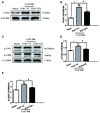
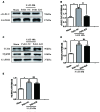
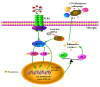
Early brain injury (EBI) following subarachnoid hemorrhage (SAH) is closely associated with neuroinflammation. Microglial activation is an early event that leads to neuroinflammation after SAH. Peli1 is an E3 ubiquitin ligase that mediates the induction of pro-inflammatory cytokines in microglia. Here we report Peli1 contributions in SAH mediated brain pathology. An SAH model was induced by endovascular perforation in adult male C57BL/6J mice. Peli1 was markedly induced in mice brains in a time-dependent manner and was predominantly expressed in CD16/32-positive microglia after SAH. Using genetic approaches, we demonstrated that decreased Peli1 significantly improved neurological deficits, attenuated brain edema, reduced over-expression of pro-inflammatory cytokine IL-6 and modified apoptotic/antiapoptotic biomarkers. In addition, Peli1 downregulation suppressed ERK and JNK phosphorylation levels via the downregulation of cIAP1/2 expression, subsequently reducing inducible nitric oxide synthase (iNOS) expression after SAH. Therefore, these findings demonstrate that Peli1 contributes to microglia-mediated neuroinflammation in EBI by mediating cIAP1/2 activation, thus promoting the activation of MyD88-dependent MAPK pathway after experimental SAH. Our findings also showed that Peli1 could promote the expression of M1 microglia polarization biomarker CD16/32 and iNOS after SAH. Targeting Peli1 exerts neuroprotective effects during EBI after SAH, thus could provide potential option for prevention-therapy in high-risk individuals.
Keywords: Peli1; early brain injury; microglia; neuroinflammation; subarachnoid hemorrhage.
Figures
Similar articles
-
Microglia activation, classification and microglia-mediated neuroinflammatory modulators in subarachnoid hemorrhage.Neural Regen Res. 2022 Jul;17(7):1404-1411. doi: 10.4103/1673-5374.330589. Neural Regen Res. 2022. PMID: 34916410 Free PMC article. Review.
-
Biglycan regulates neuroinflammation by promoting M1 microglial activation in early brain injury after experimental subarachnoid hemorrhage.J Neurochem. 2020 Feb;152(3):368-380. doi: 10.1111/jnc.14926. Epub 2019 Dec 15. J Neurochem. 2020. PMID: 31778579
-
Targeting iNOS Alleviates Early Brain Injury After Experimental Subarachnoid Hemorrhage via Promoting Ferroptosis of M1 Microglia and Reducing Neuroinflammation.Mol Neurobiol. 2022 May;59(5):3124-3139. doi: 10.1007/s12035-022-02788-5. Epub 2022 Mar 9. Mol Neurobiol. 2022. PMID: 35262869
-
Apolipoprotein E Deficiency Aggravates Neuronal Injury by Enhancing Neuroinflammation via the JNK/c-Jun Pathway in the Early Phase of Experimental Subarachnoid Hemorrhage in Mice.Oxid Med Cell Longev. 2019 Dec 26;2019:3832648. doi: 10.1155/2019/3832648. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31949876 Free PMC article.
-
Emerging Role of Microglia-Mediated Neuroinflammation in Epilepsy after Subarachnoid Hemorrhage.Mol Neurobiol. 2021 Jun;58(6):2780-2791. doi: 10.1007/s12035-021-02288-y. Epub 2021 Jan 26. Mol Neurobiol. 2021. PMID: 33501625 Review.
Cited by
-
Modulators of microglia activation and polarization in ischemic stroke (Review).Mol Med Rep. 2020 May;21(5):2006-2018. doi: 10.3892/mmr.2020.11003. Epub 2020 Feb 26. Mol Med Rep. 2020. PMID: 32323760 Free PMC article. Review.
-
Microglia activation, classification and microglia-mediated neuroinflammatory modulators in subarachnoid hemorrhage.Neural Regen Res. 2022 Jul;17(7):1404-1411. doi: 10.4103/1673-5374.330589. Neural Regen Res. 2022. PMID: 34916410 Free PMC article. Review.
-
The protective effect of the TSPO ligands 2,4-Di-Cl-MGV-1, CB86, and CB204 against LPS-induced M1 pro-inflammatory activation of microglia.Brain Behav Immun Health. 2020 May 21;5:100083. doi: 10.1016/j.bbih.2020.100083. eCollection 2020 May. Brain Behav Immun Health. 2020. PMID: 34589858 Free PMC article.
-
The blood-brain barrier and the neurovascular unit in subarachnoid hemorrhage: molecular events and potential treatments.Fluids Barriers CNS. 2022 Apr 11;19(1):29. doi: 10.1186/s12987-022-00312-4. Fluids Barriers CNS. 2022. PMID: 35410231 Free PMC article. Review.
-
A Systematic Review of Inflammatory Cytokine Changes Following Aneurysmal Subarachnoid Hemorrhage in Animal Models and Humans.Transl Stroke Res. 2022 Dec;13(6):881-897. doi: 10.1007/s12975-022-01001-y. Epub 2022 Mar 9. Transl Stroke Res. 2022. PMID: 35260989
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

