Propofol Exposure in Early Life Induced Developmental Impairments in the Mouse Cerebellum
- PMID: 29249940
- PMCID: PMC5715384
- DOI: 10.3389/fncel.2017.00373
Propofol Exposure in Early Life Induced Developmental Impairments in the Mouse Cerebellum
Abstract
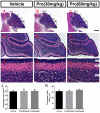
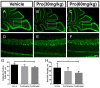
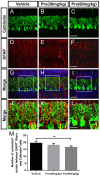
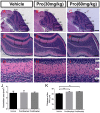
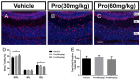
Propofol is a widely used anesthetic in the clinic while several studies have demonstrated that propofol exposure may cause neurotoxicity in the developing brain. However, the effects of early propofol exposure on cerebellar development are not well understood. Propofol (30 or 60 mg/kg) was administered to mice on postnatal day (P)7; Purkinje cell dendritogenesis and Bergmann glial cell development were evaluated on P8, and granule neuron migration was analyzed on P10. The results indicated that exposure to propofol on P7 resulted in a significant reduction in calbindin-labeled Purkinje cells and their dendrite length. Furthermore, propofol induced impairments in Bergmann glia development, which might be involved in the delay of granule neuron migration from the external granular layer (EGL) to the internal granular layer (IGL) during P8 to P10 at the 60 mg/kg dosage, but not at the 30 mg/kg dosage. Several reports have suggested that the Notch signaling pathway plays instructive roles in the morphogenesis of Bergmann glia. Here, it was revealed that propofol treatment decreased Jagged1 and Notch1 protein levels in the cerebellum on P8. Taken together, exposure to propofol during the neonatal period impairs Bergmann glia development and may therefore lead to cerebellum development defects. Our results may aid in the understanding of the neurotoxic effects of propofol when administrated to infants.
Keywords: cerebellum; development; mouse; neurotoxicity; propofol.
Figures



Similar articles
-
Activation of liver X receptor is protective against ethanol-induced developmental impairment of Bergmann glia and Purkinje neurons in the mouse cerebellum.Mol Neurobiol. 2014 Feb;49(1):176-86. doi: 10.1007/s12035-013-8510-y. Epub 2013 Jul 31. Mol Neurobiol. 2014. PMID: 23900741
-
Transcriptional Regulator ZEB2 Is Essential for Bergmann Glia Development.J Neurosci. 2018 Feb 7;38(6):1575-1587. doi: 10.1523/JNEUROSCI.2674-17.2018. Epub 2018 Jan 11. J Neurosci. 2018. PMID: 29326173 Free PMC article.
-
Spatio-temporal characterization of the pleiotrophinergic system in mouse cerebellum: evidence for its key role during ontogenesis.Exp Neurol. 2013 Sep;247:537-51. doi: 10.1016/j.expneurol.2013.02.004. Epub 2013 Feb 20. Exp Neurol. 2013. PMID: 23454176
-
Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells.Anat Sci Int. 2002 Jun;77(2):94-108. doi: 10.1046/j.0022-7722.2002.00021.x. Anat Sci Int. 2002. PMID: 12418089 Review.
-
Origin, lineage and function of cerebellar glia.Prog Neurobiol. 2013 Oct;109:42-63. doi: 10.1016/j.pneurobio.2013.08.001. Epub 2013 Aug 25. Prog Neurobiol. 2013. PMID: 23981535 Review.
Cited by
-
The Effects of General Anesthetics on Synaptic Transmission.Curr Neuropharmacol. 2020;18(10):936-965. doi: 10.2174/1570159X18666200227125854. Curr Neuropharmacol. 2020. PMID: 32106800 Free PMC article. Review.
-
AAV Delivery of shRNA Against TRPC6 in Mouse Hippocampus Impairs Cognitive Function.Front Cell Dev Biol. 2021 Jul 13;9:688655. doi: 10.3389/fcell.2021.688655. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34327201 Free PMC article.
-
Early Development of the GABAergic System and the Associated Risks of Neonatal Anesthesia.Int J Mol Sci. 2021 Nov 30;22(23):12951. doi: 10.3390/ijms222312951. Int J Mol Sci. 2021. PMID: 34884752 Free PMC article. Review.
-
17β-Estradiol Protects Neural Stem/Progenitor Cells Against Ketamine-Induced Injury Through Estrogen Receptor β Pathway.Front Neurosci. 2020 Sep 30;14:576813. doi: 10.3389/fnins.2020.576813. eCollection 2020. Front Neurosci. 2020. PMID: 33100963 Free PMC article.
-
Limb Remote Ischemic Preconditioning Reduces Repeated Ketamine Exposure-Induced Adverse Effects in the Developing Brain of Rats.J Mol Neurosci. 2019 May;68(1):58-65. doi: 10.1007/s12031-019-01282-3. Epub 2019 Mar 7. J Mol Neurosci. 2019. PMID: 30847723
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

