Lithium Suppresses Hedgehog Signaling via Promoting ITCH E3 Ligase Activity and Gli1-SUFU Interaction in PDA Cells
- PMID: 29249966
- PMCID: PMC5715333
- DOI: 10.3389/fphar.2017.00820
Lithium Suppresses Hedgehog Signaling via Promoting ITCH E3 Ligase Activity and Gli1-SUFU Interaction in PDA Cells
Abstract
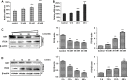
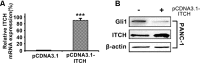
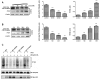
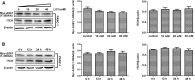
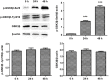
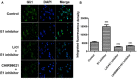
Dysregulation of Hedgehog (Hh) signaling pathway is one of the hallmarks of pancreatic ductal adenocarcinoma (PDA). Lithium, a clinical mood stabilizer for the treatment of mental disorders, is known to suppress tumorigenic potential of PDA cells by targeting the Hh/Gli signaling pathway. In this study, we investigated the molecular mechanism of lithium induced down-regulation of Hh/Gli1. Our data show that lithium promotes the poly-ubiquitination and proteasome-mediated degradation of Gli1 through activating E3 ligase ITCH. Additionally, lithium enhances interaction between Gli1 and SUFU via suppressing GSK3β, which phosphorylates SUFU and destabilizes the SUFU-Gli1 inhibitory complex. Our studies illustrate a novel mechanism by which lithium suppresses Hh signaling via simultaneously promoting ITCH-dependent Gli1 ubiquitination/degradation and SUFU-mediated Gli1 inhibition.
Keywords: GSK3β; Gli1; ITCH; hedgehog signaling; lithium; pancreatic cancer; ubiquitination.
Figures

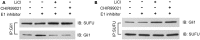
Similar articles
-
Lithium inhibits tumorigenic potential of PDA cells through targeting hedgehog-GLI signaling pathway.PLoS One. 2013 Apr 23;8(4):e61457. doi: 10.1371/journal.pone.0061457. Print 2013. PLoS One. 2013. PMID: 23626687 Free PMC article.
-
SUFU promotes GLI activity in a Hedgehog-independent manner in pancreatic cancer.Biochem J. 2023 Aug 16;480(15):1199-1216. doi: 10.1042/BCJ20220439. Biochem J. 2023. PMID: 37477952 Free PMC article.
-
SCL/TAL1 interrupting locus derepresses GLI1 from the negative control of suppressor-of-fused in pancreatic cancer cell.Cancer Res. 2008 Oct 1;68(19):7723-9. doi: 10.1158/0008-5472.CAN-07-6661. Cancer Res. 2008. PMID: 18829525
-
Targeting Hedgehog Signalling through the Ubiquitylation Process: The Multiple Roles of the HECT-E3 Ligase Itch.Cells. 2019 Jan 29;8(2):98. doi: 10.3390/cells8020098. Cells. 2019. PMID: 30699938 Free PMC article. Review.
-
Hedgehog/GLI Signaling Pathway: Transduction, Regulation, and Implications for Disease.Cancers (Basel). 2021 Jul 7;13(14):3410. doi: 10.3390/cancers13143410. Cancers (Basel). 2021. PMID: 34298625 Free PMC article. Review.
Cited by
-
The Role of HECT-Type E3 Ligase in the Development of Cardiac Disease.Int J Mol Sci. 2021 Jun 4;22(11):6065. doi: 10.3390/ijms22116065. Int J Mol Sci. 2021. PMID: 34199773 Free PMC article. Review.
-
RNA-seq and ChIP-seq Identification of Unique and Overlapping Targets of GLI Transcription Factors in Melanoma Cell Lines.Cancers (Basel). 2022 Sep 19;14(18):4540. doi: 10.3390/cancers14184540. Cancers (Basel). 2022. PMID: 36139698 Free PMC article.
-
Impact of posttranslational modifications in pancreatic carcinogenesis and treatments.Cancer Metastasis Rev. 2021 Sep;40(3):739-759. doi: 10.1007/s10555-021-09980-4. Epub 2021 Aug 3. Cancer Metastasis Rev. 2021. PMID: 34342796 Review.
-
Regulation of Hedgehog Signal Transduction by Ubiquitination and Deubiquitination.Int J Mol Sci. 2021 Dec 11;22(24):13338. doi: 10.3390/ijms222413338. Int J Mol Sci. 2021. PMID: 34948134 Free PMC article. Review.
-
Signaling Switching from Hedgehog-GLI to MAPK Signaling Potentially Serves as a Compensatory Mechanism in Melanoma Cell Lines Resistant to GANT-61.Biomedicines. 2023 May 3;11(5):1353. doi: 10.3390/biomedicines11051353. Biomedicines. 2023. PMID: 37239024 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

