Potential Contributions of miR-200a/-200b and Their Target Gene-Leptin to the Sexual Size Dimorphism in Yellow Catfish
- PMID: 29249979
- PMCID: PMC5714929
- DOI: 10.3389/fphys.2017.00970
Potential Contributions of miR-200a/-200b and Their Target Gene-Leptin to the Sexual Size Dimorphism in Yellow Catfish
Abstract
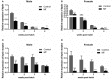
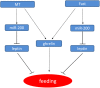
Sexual size dimorphism is the consequence of differential expression of sex-biased genes related to feeding and growth. Leptin is known to regulate energy balance by regulating food intake. In order to investigate the molecular mechanism of sexual size dimorphism in yellow catfish (Pelteobagrus fulvidraco), the expression of leptin (lep) and its functional receptor (lepr) were detected during larval development. Both lep and lepr have lower expression in males than in females during 1-4 weeks post hatching. 17a-Methyltestosterone (MT) treatment resulted in decreased expression of lep and lepr in both male and female larval fish. Interestingly, the mRNA levels of lep and lepr in juvenile male were significantly decreased compared with juvenile female during short-term fasting periods. Lep was predicted to be a potential target of miR-200a and miR-200b that had an opposite expression pattern to lep in male and female larvas. The results of luciferase reporter assay suggested that lep is a target of miR-200a/-200b. Subsequently, male hormone and fasting treatment have opposite effects on the expression of miR-200a/-200b and lep between males and females. In summary, our results suggest that sexual size dimorphism in fish species is probably caused by the sexually dimorphic expression of leptin, which could be negatively regulated by miR-200a/-200b.
Keywords: fasting; leptin; miR-200a/b; sex hormone; sexual dimorphism.
Figures





References
-
- Beardmore J. A., Mair G. C., Lewis R. I. (2001). Monosex male production in finfish as exemplified by tilapia: applications, problems, and prospects. Aquaculture 197, 283–301. 10.1016/S0044-8486(01)00590-7 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

