Stenotrophomonas maltophilia as an Emerging Ubiquitous Pathogen: Looking Beyond Contemporary Antibiotic Therapy
- PMID: 29250041
- PMCID: PMC5714879
- DOI: 10.3389/fmicb.2017.02276
Stenotrophomonas maltophilia as an Emerging Ubiquitous Pathogen: Looking Beyond Contemporary Antibiotic Therapy
Abstract
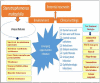
Stenotrophomonas maltophilia is a commensal and an emerging pathogen earlier noted in broad-spectrum life threatening infections among the vulnerable, but more recently as a pathogen in immunocompetent individuals. The bacteria are consistently being implicated in necrotizing otitis, cutaneous infections including soft tissue infection and keratitis, endocarditis, meningitis, acute respiratory tract infection (RTI), bacteraemia (with/without hematological malignancies), tropical pyomyositis, cystic fibrosis, septic arthritis, among others. S. maltophilia is also an environmental bacteria occurring in water, rhizospheres, as part of the animals' microflora, in foods, and several other microbiota. This review highlights clinical reports on S. maltophilia both as an opportunistic and as true pathogen. Also, biofilm formation as well as quorum sensing, extracellular enzymes, flagella, pili/fimbriae, small colony variant, other virulence or virulence-associated factors, the antibiotic resistance factors, and their implications are considered. Low outer membrane permeability, natural MDR efflux systems, and/or resistance genes, resistance mechanisms like the production of two inducible chromosomally encoded β-lactamases, and lack of carefully compiled patient history are factors that pose great challenges to the S. maltophilia control arsenals. The fluoroquinolone, some tetracycline derivatives and trimethoprim-sulphamethaxole (TMP-SMX) were reported as effective antibiotics with good therapeutic outcome. However, TMP-SMX resistance and allergies to sulfa together with high toxicity of fluoroquinolone are notable setbacks. S. maltophilia's production and sustenance of biofilm by quorum sensing enhance their virulence, resistance to antibiotics and gene transfer, making quorum quenching an imperative step in Stenotrophomonas control. Incorporating several other proven approaches like bioengineered bacteriophage therapy, Epigallocatechin-3-gallate (EGCG), essential oil, nanoemulsions, and use of cationic compounds are promising alternatives which can be incorporated in Stenotrophomonas control arsenal.
Keywords: Stenotrophomonas maltophilia; phage therapy; quorum quenching; resistance genes; sulfa.
Figures

References
-
- Adegoke A. A., Okoh A. I. (2012). Commensal Staphylococcus spp., Acinetobacter spp. and Stenotrophomonas maltophilia as reservoirs of antibiotic resistance genes. Afr. J. Biotechnol. 11, 12429–12435. 10.5897/AJB12.141 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

