Down-Regulation of OsEMF2b Caused Semi-sterility Due to Anther and Pollen Development Defects in Rice
- PMID: 29250087
- PMCID: PMC5715369
- DOI: 10.3389/fpls.2017.01998
Down-Regulation of OsEMF2b Caused Semi-sterility Due to Anther and Pollen Development Defects in Rice
Abstract
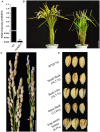
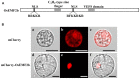
Anther and pollen development are crucial processes of plant male reproduction. Although a number of genes involved in these processes have been identified, the regulatory networks of pollen and anther development are still unclear. EMBRYONIC FLOWER 2b (OsEMF2b) is important for rice development. Its biological function in floral organ, flowering time and meristem determinacy have been well-studied, but its role, if only, on male reproduction is still unknown, because null mutants of OsEMF2b barely have anthers. In this study, we identified a weak allele of OsEMF2b, osemf2b-4. The T-DNA insertion was located in the promoter region of OsEMF2b, and OsEMF2b expression was significantly decreased in osemf2b-4. The osemf2b-4 mutant exhibited much more normal anthers than null mutants of OsEMF2b, and also showed defective floret development similar to null mutants. Cytological analysis showed various defects of anther wall and pollen development in osemf2b-4, such as slightly or extremely enlarged tapetum, irregular or normal morphology microspores, and partial or complete sterility. OsEMF2b was highly expressed in tapetum and microspores, and the protein was localized in the nucleus. The expression of 15 genes essential for anther and pollen development was investigated in both WT and osemf2b-4. Fourteen genes including GAMYB was up-regulated, and only PTC1 was down-regulated in osemf2b-4. This suggests that up-regulated GAMYB and down-regulated PTC1 might contribute to the defective anther and pollen development in osemf2b-4. Overall, our work suggests that OsEMF2b plays an essential role during post-meiotic anther and pollen development.
Keywords: OsEMF2b; anther development; male reproductive development; rice; semi-sterility.
Figures




Similar articles
-
Tapetum and middle layer control male fertility in Actinidia deliciosa.Ann Bot. 2013 Oct;112(6):1045-55. doi: 10.1093/aob/mct173. Epub 2013 Aug 21. Ann Bot. 2013. PMID: 23965617 Free PMC article.
-
Cytological and transcriptome analyses reveal OsPUB73 defect affects the gene expression associated with tapetum or pollen exine abnormality in rice.BMC Plant Biol. 2019 Dec 10;19(1):546. doi: 10.1186/s12870-019-2175-2. BMC Plant Biol. 2019. PMID: 31823718 Free PMC article.
-
OsGPAT3 Plays a Critical Role in Anther Wall Programmed Cell Death and Pollen Development in Rice.Int J Mol Sci. 2018 Dec 12;19(12):4017. doi: 10.3390/ijms19124017. Int J Mol Sci. 2018. PMID: 30545137 Free PMC article.
-
Thermal stress impacts reproductive development and grain yield in rice.Plant Physiol Biochem. 2017 Jun;115:57-72. doi: 10.1016/j.plaphy.2017.03.011. Epub 2017 Mar 16. Plant Physiol Biochem. 2017. PMID: 28324683 Review.
-
Engineered Male Sterility by Early Anther Ablation Using the Pea Anther-Specific Promoter PsEND1.Front Plant Sci. 2019 Jun 25;10:819. doi: 10.3389/fpls.2019.00819. eCollection 2019. Front Plant Sci. 2019. PMID: 31293612 Free PMC article. Review.
Cited by
-
Lack of the α1,3-Fucosyltransferase Gene (Osfuct) Affects Anther Development and Pollen Viability in Rice.Int J Mol Sci. 2018 Apr 18;19(4):1225. doi: 10.3390/ijms19041225. Int J Mol Sci. 2018. PMID: 29670011 Free PMC article.
-
The MYB transcription factor Baymax1 plays a critical role in rice male fertility.Theor Appl Genet. 2021 Feb;134(2):453-471. doi: 10.1007/s00122-020-03706-w. Epub 2020 Oct 21. Theor Appl Genet. 2021. PMID: 33089345
-
A VIN3-like Protein OsVIL1 Is Involved in Grain Yield and Biomass in Rice.Plants (Basel). 2021 Dec 28;11(1):83. doi: 10.3390/plants11010083. Plants (Basel). 2021. PMID: 35009085 Free PMC article.
-
A Novel Embryo Phenotype Associated With Interspecific Hybrid Weakness in Rice Is Controlled by the MADS-Domain Transcription Factor OsMADS8.Front Plant Sci. 2022 Jan 5;12:778008. doi: 10.3389/fpls.2021.778008. eCollection 2021. Front Plant Sci. 2022. PMID: 35069634 Free PMC article.
-
Genome-Wide Identification and Analysis of the Polycomb Group Family in Medicago truncatula.Int J Mol Sci. 2021 Jul 14;22(14):7537. doi: 10.3390/ijms22147537. Int J Mol Sci. 2021. PMID: 34299158 Free PMC article.
References
-
- Cao H., Li X., Wang Z., Ding M., Sun Y., Dong F., et al. (2015). Histone H2B monoubiquitination mediated by HISTONE MONOUBIQUITINATION1 and HISTONE MONOUBIQUITINATION2 Is involved in anther development by regulating tapetum degradation-related genes in rice. Plant Physiol. 168 1389–1405. 10.1104/pp.114.256578 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

