Nitro-sonium complexation by the tetra-phospho-nate cavitand 5,11,17,23-tetra-methyl-6,10:12,16:18,22:24,4-tetra-kis-(phenyl-phospho-nato-κ2O, O)resorcin(4)arene
- PMID: 29250390
- PMCID: PMC5730227
- DOI: 10.1107/S2056989017015857
Nitro-sonium complexation by the tetra-phospho-nate cavitand 5,11,17,23-tetra-methyl-6,10:12,16:18,22:24,4-tetra-kis-(phenyl-phospho-nato-κ2O, O)resorcin(4)arene
Abstract
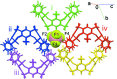
The crystal structure of a new supra-molecular complex between the tetra-phos-pho-nate cavitand 5,11,17,23-tetra-methyl-6,10:12,16:18,22:24,4-tetra-kis(phenyl-phospho-nato-κ2O,O')resorcin(4)arene and the nitrosyl cation NO+, as the BF4- salt, is reported. The complex, of general formula [(C56H44P4O12)(NO)]BF4·CH2Cl2 or NO@Tiiii[H, CH3, C6H5] BF4·CH2Cl2, crystallizes in the space group P-1. The nitrosyl cation is disordered over two equivalent positions, with occupancies of 0.503 (2) and 0.497 (2), and inter-acts with two adjacent P=O groups at the upper rim of the cavitand through dipole-charge inter-actions. In the lattice, the cavitands are connected through a series of C-H⋯π inter-actions involving the methyl and methyl-enic H atoms and the aromatic rings of the macrocycle. The structure is further stabilized by the presence of C-H⋯F inter-actions between the hydrogen atoms of the cavitands and the F atoms of the tetra-fluorido-borate anion. As a result of the disorder, the lattice di-chloro-methane mol-ecules could not be modelled in terms of atomic sites, and were treated using the PLATON SQUEEZE procedure [Spek (2015 ▸). Acta Cryst. C71, 9-18]. The complexation process has also been studied in solution through NMR titrations.
Keywords: C—H⋯F interactions; C—H⋯π interactions; crystal structure; inclusion compounds; nitrosonium ion; tetraphosphonate cavitands.
Figures



References
-
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst. 32, 115–119.
-
- Botta, B., D’Acquarica, I., Delle Monache, G., Nevola, L., Tullo, D., Ugozzoli, F. & Pierini, M. (2007). J. Am. Chem. Soc. 129, 11202–11212. - PubMed
-
- Bruker (2008). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Cram, D. J. (1983). Science, 219, 1177–1183. - PubMed
-
- Cram, D. J. & Cram, J. M. (1994). Container Molecules and their Guests, Monographs in Supramolecular Chemistry, edited by J. F. Stoddart, vol. 4. Royal Society of Chemistry, Cambridge, UK.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
