Mitotic Regulation by NEK Kinase Networks
- PMID: 29250521
- PMCID: PMC5716973
- DOI: 10.3389/fcell.2017.00102
Mitotic Regulation by NEK Kinase Networks
Abstract
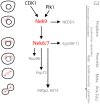
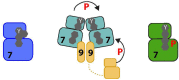
Genetic studies in yeast and Drosophila led to identification of cyclin-dependent kinases (CDKs), Polo-like kinases (PLKs) and Aurora kinases as essential regulators of mitosis. These enzymes have since been found in the majority of eukaryotes and their cell cycle-related functions characterized in great detail. However, genetic studies in another fungal species, Aspergillus nidulans, identified a distinct family of protein kinases, the NEKs, that are also widely conserved and have key roles in the cell cycle, but which remain less well studied. Nevertheless, it is now clear that multiple NEK family members act in networks to regulate specific events of mitosis, including centrosome separation, spindle assembly and cytokinesis. Here, we describe our current understanding of how the NEK kinases contribute to these processes, particularly through targeted phosphorylation of proteins associated with the microtubule cytoskeleton. We also present the latest findings on molecular events that control the activation state of the NEKs and how these are revealing novel modes of enzymatic regulation relevant not only to other kinases but also to pathological mechanisms of disease.
Keywords: centrosome; cilia; microtubule; mitosis; protein kinase.
Figures

References
-
- Alexander J., Lim D., Joughin B. A., Hegemann B., Hutchins J. R., Ehrenberger T., et al. . (2011). Spatial exclusivity combined with positive and negative selection of phosphorylation motifs is the basis for context-dependent mitotic signaling. Sci. Signal 4:ra42. 10.1126/scisignal.2001796 - DOI - PMC - PubMed
Publication types
Grants and funding
- MR/L017032/1/MRC_/Medical Research Council/United Kingdom
- BB/F010702/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 16-0119/AICR_/Worldwide Cancer Research/United Kingdom
- 13-0042/AICR_/Worldwide Cancer Research/United Kingdom
- MR/L017032/2/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

