Beta-Defensin-2 and Beta-Defensin-3 Reduce Intestinal Damage Caused by Salmonella typhimurium Modulating the Expression of Cytokines and Enhancing the Probiotic Activity of Enterococcus faecium
- PMID: 29250559
- PMCID: PMC5700477
- DOI: 10.1155/2017/6976935
Beta-Defensin-2 and Beta-Defensin-3 Reduce Intestinal Damage Caused by Salmonella typhimurium Modulating the Expression of Cytokines and Enhancing the Probiotic Activity of Enterococcus faecium
Abstract
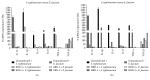
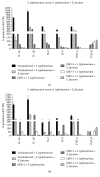
The intestinal microbiota is a major factor in human health and disease. This microbial community includes autochthonous (permanent inhabitants) and allochthonous (transient inhabitants) microorganisms that contribute to maintaining the integrity of the intestinal wall, modulating responses to pathogenic noxae and representing a key factor in the maturation of the immune system. If this healthy microbiota is disrupted by antibiotics, chemotherapy, or a change in diet, intestinal colonization by pathogenic bacteria or viruses may occur, leading to disease. To manage substantial microbial exposure, epithelial surfaces of the intestinal tract produce a diverse arsenal of antimicrobial peptides (AMPs), including, of considerable importance, the β-defensins, which directly kill or inhibit the growth of microorganisms. Based on the literature data, the purpose of this work was to create a line of intestinal epithelial cells able to stably express gene encoding human β-defensin-2 (hBD-2) and human β-defensin-3 (hBD-3), in order to test their role in S. typhimurium infections and their interaction with the bacteria of the gut microbiota.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

