Glucose-Stimulated Insulin Response of Silicon Nanopore-Immunoprotected Islets under Convective Transport
- PMID: 29250596
- PMCID: PMC5729757
- DOI: 10.1021/acsbiomaterials.6b00814
Glucose-Stimulated Insulin Response of Silicon Nanopore-Immunoprotected Islets under Convective Transport
Abstract
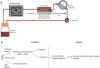
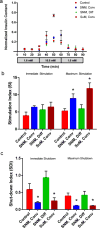
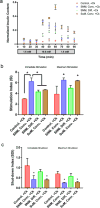
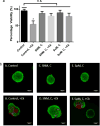
Major clinical challenges associated with islet transplantation for type 1 diabetes include shortage of donor organs, poor engraftment due to ischemia, and need for immunosuppressive medications. Semipermeable membrane capsules can immunoprotect transplanted islets by blocking passage of the host's immune components while providing exchange of glucose, insulin, and other small molecules. However, capsules-based diffusive transport often exacerbates ischemic injury to islets by reducing the rate of oxygen and nutrient transport. We previously reported the efficacy of a newly developed semipermeable ultrafiltration membrane, the silicon nanopore membrane (SNM) under convective-driven transport, in limiting the passage of pro-inflammatory cytokines while overcoming the mass transfer limitations associated with diffusion through nanometer-scale pores. In this study, we report that SNM-encapsulated mouse islets perfused in culture solution under convection outperformed those under diffusive conditions in terms of magnitude (1.49-fold increase in stimulation index and 3.86-fold decrease in shutdown index) and rate of insulin secretion (1.19-fold increase and 6.45-fold decrease during high and low glucose challenges), respectively. Moreover, SNM-encapsulated mouse islets under convection demonstrated rapid glucose-insulin sensing within a physiologically relevant time-scale while retaining healthy islet viability even under cytokine exposure. We conclude that encapsulation of islets with SNM under convection improves islet in vitro functionality. This approach may provide a novel strategy for islet transplantation in the clinical setting.
Keywords: convection; diffusion; glucose-insulin kinetics; immunoisolation; silicon nanopore membranes (SNM).
Conflict of interest statement
Notes The authors declare the following competing financial interest(s): Dr. Shuvo Roy is a co-founder of Silicon Kidney, LLC. There are no competing interests or conflicts of interest related to the work presented in this manuscript for all authors.
Figures
References
-
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8. - PubMed
-
- Gruessner AC, Sutherland DE, Gruessner RW. Pancreas transplantation in the United States: a review. Curr Opin Organ Transplant. 2010;15(1):93–101. - PubMed
-
- Hirshberg B, Rother KI, Digon BJ, 3rd, Lee J, Gaglia JL, Hines K, Read EJ, Chang R, Wood BJ, Harlan DM. Benefits and risks of solitary islet transplantation for type 1 diabetes using steroid-sparing immunosuppression: the National Institutes of Health experience. Diabetes Care. 2003;26(12):3288–95. - PubMed
-
- Markmann JF, Deng S, Huang X, Desai NM, Velidedeoglu EH, Lui C, Frank A, Markmann E, Palanjian M, Brayman K, Wolf B, Bell E, Vitamaniuk M, Doliba N, Matschinsky F, Barker CF, Naji A. Insulin independence following isolated islet transplantation and single islet infusions. Ann Surg. 2003;237(6):741–9. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
