Ultrastructure of neurovascular changes in human diabetic retinopathy
- PMID: 29251013
- PMCID: PMC5849217
- DOI: 10.1177/0394632017748841
Ultrastructure of neurovascular changes in human diabetic retinopathy
Abstract
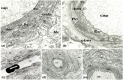
The previous concept regarding diabetic retinopathy assigned a primary role to hyperglycemia-induced microvascular alterations, while neuronal and glial abnormalities were considered to be secondary to either ischemia or exudation. The aim of this study was to reveal the potential role of neuronal and glial cells in initial and advanced alterations of the retinopathy in human type 2 diabetes. Electron microscopy and histochemical studies were performed on 38 surgically removed human eyes (28 obtained from diabetic patients and 10 from non-diabetic patients). Morphometric analysis of basement membrane material and lipids was performed. An accumulation of metabolic by-products was found in the capillary wall with aging: this aspect was significantly more pronounced in diabetics. Müller glial cells were found to contribute to alterations of the capillary wall and to occlusion, as well as to the development of proliferative retinopathy and cystoid degeneration of the retina. Our results showed morphological evidence regarding the role of neuronal and glial cells in the pathology of diabetic retinopathy, prior and in addition to microangiopathy. These morphological findings support a neurovascular pathogenesis at the origin of diabetic retinopathy, thus the current treatment approach should be completed by neuroprotective measures.
Keywords: basement membrane; diabetic macular edema; diabetic retinopathy; glia; proliferative diabetic retinopathy.
Conflict of interest statement
Figures


References
-
- Barber AJ. (2015) Diabetic retinopathy: Recent advances towards understanding neurodegeneration and vision loss. Science China Life Sciences 58(6): 541–549. - PubMed
-
- Jonsson KB, Frydkjaer-Olsen U, Grauslund J. (2016) Vascular changes and neurodegeneration in the early stages of diabetic retinopathy: Which comes first? Ophthalmic Research 56(1): 1–9. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

