Microfluidic platforms for the study of neuronal injury in vitro
- PMID: 29251352
- PMCID: PMC5831486
- DOI: 10.1002/bit.26519
Microfluidic platforms for the study of neuronal injury in vitro
Abstract
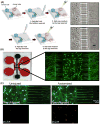
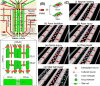
Traumatic brain injury (TBI) affects 5.3 million people in the United States, and there are 12,500 new cases of spinal cord injury (SCI) every year. There is yet a significant need for in vitro models of TBI and SCI in order to understand the biological mechanisms underlying central nervous system (CNS) injury and to identify and test therapeutics to aid in recovery from neuronal injuries. While TBI or SCI studies have been aided with traditional in vivo and in vitro models, the innate limitations in specificity of injury, isolation of neuronal regions, and reproducibility of these models can decrease their usefulness in examining the neurobiology of injury. Microfluidic devices provide several advantages over traditional methods by allowing researchers to (1) examine the effect of injury on specific neural components, (2) fluidically isolate neuronal regions to examine specific effects on subcellular components, and (3) reproducibly create a variety of injuries to model TBI and SCI. These microfluidic devices are adaptable for modeling a wide range of injuries, and in this review, we will examine different methodologies and models recently utilized to examine neuronal injury. Specifically, we will examine vacuum-assisted axotomy, physical injury, chemical injury, and laser-based axotomy. Finally, we will discuss the benefits and downsides to each type of injury model and discuss how researchers can use these parameters to pick a particular microfluidic device to model CNS injury.
Keywords: axotomy; chemical and physical neuronal injury; microfluidic neuronal culture; spinal cord injury; traumatic brain injury; vacuum-assisted and laser-based neuronal injury.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures





References
-
- Benam KH, Dauth S, Hassell B, Herland A, Jain A, Jang K-J, Karalis K, Kim HJ, MacQueen L, Mahmoodian R. Engineered in vitro disease models. Annual Review of Pathology: Mechanisms of Disease. 2015;10:195–262. - PubMed
-
- Center NSCIS. Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham; 2015. pp. 1–2.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

