Lipotoxicity induces hepatic protein inclusions through TANK binding kinase 1-mediated p62/sequestosome 1 phosphorylation
- PMID: 29251796
- PMCID: PMC6005718
- DOI: 10.1002/hep.29742
Lipotoxicity induces hepatic protein inclusions through TANK binding kinase 1-mediated p62/sequestosome 1 phosphorylation
Abstract
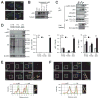
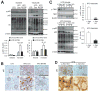
Obesity commonly leads to hepatic steatosis, which often provokes lipotoxic injuries to hepatocytes that cause nonalcoholic steatohepatitis (NASH). NASH, in turn, is associated with the accumulation of insoluble protein aggregates that are composed of ubiquitinated proteins and ubiquitin adaptor p62/sequestosome 1 (SQSTM1). Formation of p62 inclusions in hepatocytes is the critical marker that distinguishes simple fatty liver from NASH and predicts a poor prognostic outcome for subsequent liver carcinogenesis. However, the molecular mechanism by which lipotoxicity induces protein aggregation is currently unknown. Here, we show that, upon saturated fatty acid-induced lipotoxicity, TANK binding kinase 1 (TBK1) is activated and phosphorylates p62. TBK1-mediated p62 phosphorylation is important for lipotoxicity-induced aggregation of ubiquitinated proteins and formation of large protein inclusions in hepatocytes. In addition, cyclic GMP-AMP synthase (cGAS) and stimulator of interferon genes (STING), upstream regulators of TBK1, are involved in lipotoxic activation of TBK1 and subsequent p62 phosphorylation in hepatocytes. Furthermore, TBK1 inhibition prevented formation of ubiquitin-p62 aggregates not only in cultured hepatocytes, but also in mouse models of obesity and NASH.
Conclusion: These results suggest that lipotoxic activation of TBK1 and subsequent p62 phosphorylation are critical steps in the NASH pathology of protein inclusion accumulation in hepatocytes. This mechanism can provide an explanation for how hypernutrition and obesity promote the development of severe liver pathologies, such as steatohepatitis and liver cancer, by facilitating the formation of p62 inclusions. (Hepatology 2018).
© 2017 by the American Association for the Study of Liver Diseases.
Figures






References
-
- Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725. e716. - PubMed
-
- Strnad P, Nuraldeen R, Guldiken N, Hartmann D, Mahajan V, Denk H, Haybaeck J. Broad spectrum of hepatocyte inclusions in humans, animals, and experimental models. Compr Physiol. 2013;3:1393–1436. - PubMed
-
- Kucukoglu O, Guldiken N, Chen Y, Usachov V, El-Heliebi A, Haybaeck J, Denk H, et al. High-fat diet triggers Mallory-Denk body formation through misfolding and crosslinking of excess keratin 8. Hepatology. 2014;60:169–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

