Blood-brain barrier development: Systems modeling and predictive toxicology
- PMID: 29251840
- PMCID: PMC6476421
- DOI: 10.1002/bdr2.1180
Blood-brain barrier development: Systems modeling and predictive toxicology
Abstract
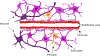
The blood-brain barrier (BBB) serves as a gateway for passage of drugs, chemicals, nutrients, metabolites, and hormones between vascular and neural compartments in the brain. Here, we review BBB development with regard to the microphysiology of the neurovascular unit (NVU) and the impact of BBB disruption on brain development. Our focus is on modeling these complex systems. Extant in silico models are available as tools to predict the probability of drug/chemical passage across the BBB; in vitro platforms for high-throughput screening and high-content imaging provide novel data streams for profiling chemical-biological interactions; and engineered human cell-based microphysiological systems provide empirical models with which to investigate the dynamics of NVU function. Computational models are needed that bring together kinetic and dynamic aspects of NVU function across gestation and under various physiological and toxicological scenarios. This integration will inform adverse outcome pathways to reduce uncertainty in translating in vitro data and in silico models for use in risk assessments that aim to protect neurodevelopmental health.
Keywords: blood-brain barrier; brain; children's environmental health; embryo; neurovascular development; neurovascular unit; predictive toxicology.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
6. Conflict of interest statement
The authors declare no conflicts.
Figures




References
-
- Abbott NJ, 2013. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 36(3), 437–449. - PubMed
-
- Abbott NJ, Lane NJ, Bundgaard M, 1992. A fibre matrix model for the restricting junction of the blood-brain barrier in a cephalopod mollusc: implications for capillary and epithelial permeability. J Neurocytol 21(4), 304–311. - PubMed
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ, 2010. Structure and function of the blood-brain barrier. Neurobiol Dis 37(1), 13–25. - PubMed
-
- Achyuta AK, Conway AJ, Crouse RB, Bannister EC, Lee RN, Katnik CP, Behensky AA, Cuevas J, Sundaram SS, 2013. A modular approach to create a neurovascular unit-on-a-chip. Lab Chip 13(4), 542–553. - PubMed
-
- Adriani G, Ma D, Pavesi A, Kamm RD, Goh EL, 2016. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood-brain barrier. Lab Chip 17(3), 448–459. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

