Microglia ablation alleviates myelin-associated catatonic signs in mice
- PMID: 29252214
- PMCID: PMC5785265
- DOI: 10.1172/JCI97032
Microglia ablation alleviates myelin-associated catatonic signs in mice
Abstract
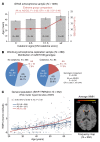
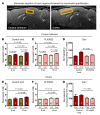
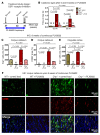
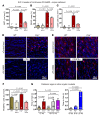
The underlying cellular mechanisms of catatonia, an executive "psychomotor" syndrome that is observed across neuropsychiatric diseases, have remained obscure. In humans and mice, reduced expression of the structural myelin protein CNP is associated with catatonic signs in an age-dependent manner, pointing to the involvement of myelin-producing oligodendrocytes. Here, we showed that the underlying cause of catatonic signs is the low-grade inflammation of white matter tracts, which marks a final common pathway in Cnp-deficient and other mutant mice with minor myelin abnormalities. The inhibitor of CSF1 receptor kinase signaling PLX5622 depleted microglia and alleviated the catatonic symptoms of Cnp mutants. Thus, microglia and low-grade inflammation of myelinated tracts emerged as the trigger of a previously unexplained mental condition. We observed a very high (25%) prevalence of individuals with catatonic signs in a deeply phenotyped schizophrenia sample (n = 1095). Additionally, we found the loss-of-function allele of a myelin-specific gene (CNP rs2070106-AA) associated with catatonia in 2 independent schizophrenia cohorts and also associated with white matter hyperintensities in a general population sample. Since the catatonic syndrome is likely a surrogate marker for other executive function defects, we suggest that microglia-directed therapies may be considered in psychiatric disorders associated with myelin abnormalities.
Keywords: Demyelinating disorders; Inflammation; Mouse models; Neuroscience; Schizophrenia.
Conflict of interest statement
Figures

Comment in
-
Micro(glial)-managing executive function: white matter inflammation drives catatonia.J Clin Invest. 2018 Feb 1;128(2):564-566. doi: 10.1172/JCI98761. Epub 2017 Dec 18. J Clin Invest. 2018. PMID: 29252213 Free PMC article.
References
-
- Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press; 2003.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

