Dexmedetomidine Prevents Cognitive Decline by Enhancing Resolution of High Mobility Group Box 1 Protein-induced Inflammation through a Vagomimetic Action in Mice
- PMID: 29252509
- PMCID: PMC6445386
- DOI: 10.1097/ALN.0000000000002038
Dexmedetomidine Prevents Cognitive Decline by Enhancing Resolution of High Mobility Group Box 1 Protein-induced Inflammation through a Vagomimetic Action in Mice
Abstract
Background: Inflammation initiated by damage-associated molecular patterns has been implicated for the cognitive decline associated with surgical trauma and serious illness. We determined whether resolution of inflammation mediates dexmedetomidine-induced reduction of damage-associated molecular pattern-induced cognitive decline.
Methods: Cognitive decline (assessed by trace fear conditioning) was induced with high molecular group box 1 protein, a damage-associated molecular pattern, in mice that also received blockers of neural (vagal) and humoral inflammation-resolving pathways. Systemic and neuroinflammation was assessed by proinflammatory cytokines.
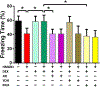
Results: Damage-associated molecular pattern-induced cognitive decline and inflammation (mean ± SD) was reversed by dexmedetomidine (trace fear conditioning: 58.77 ± 8.69% vs. 41.45 ± 7.64%, P < 0.0001; plasma interleukin [IL]-1β: 7.0 ± 2.2 pg/ml vs. 49.8 ± 6.0 pg/ml, P < 0.0001; plasma IL-6: 3.2 ± 1.6 pg/ml vs. 19.5 ± 1.7 pg/ml, P < 0.0001; hippocampal IL-1β: 4.1 ± 3.0 pg/mg vs. 41.6 ± 8.0 pg/mg, P < 0.0001; hippocampal IL-6: 3.4 ± 1.3 pg/mg vs. 16.2 ± 2.7 pg/mg, P < 0.0001). Reversal by dexmedetomidine was prevented by blockade of vagomimetic imidazoline and α7 nicotinic acetylcholine receptors but not by α2 adrenoceptor blockade. Netrin-1, the orchestrator of inflammation-resolution, was upregulated (fold-change) by dexmedetomidine (lung: 1.5 ± 0.1 vs. 0.7 ± 0.1, P < 0.0001; spleen: 1.5 ± 0.2 vs. 0.6 ± 0.2, P < 0.0001), resulting in upregulation of proresolving (lipoxin-A4: 1.7 ± 0.2 vs. 0.9 ± 0.2, P < 0.0001) and downregulation of proinflammatory (leukotriene-B4: 1.0 ± 0.2 vs. 3.0 ± 0.3, P < 0.0001) humoral mediators that was prevented by α7 nicotinic acetylcholine receptor blockade.
Conclusions: Dexmedetomidine resolves inflammation through vagomimetic (neural) and humoral pathways, thereby preventing damage-associated molecular pattern-mediated cognitive decline.
Conflict of interest statement
Conflicts of Interest
MM is a co-inventor on a patent for the use of dexmedetomidine for sedation. Between 1987–1991 MM’s laboratory at Stanford University received $250,000 for the assignment of the patent to Farmos, the company that synthesized dexmedetomidine. Between 1995–2008, MM was intermittently paid as a consultant by Orion-Farmos, Abbott Labs and Hospira for advising on the pivotal Phase III clinical trials, approval of the New Drug Application, and for subsequent marketing of the product. MM has not received any payments for at least the last 5 years. MM has not and will not receive royalty payments for sales of dexmedetomidine.
Figures

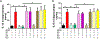

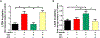

References
-
- Forsberg A, Cervenka S, Fagerlun MJ, Rasmussen L, Zetterberg H, Harris HE, Stridh P, Christensson E, Granstrom A, Schening A, Dymmel K, Knave N, Terrando N, Maze M, Borg J, A. V, Varrone A, Halldin C, Blennow K, Farde L, Eriksson L: The immune response of the human brain to abdominal surgery. Ann Neurol 2017; 84: 572–82 - PubMed

