Hypertonic saline attenuates the cytokine-induced pro-inflammatory signature in primary human lung epithelia
- PMID: 29253007
- PMCID: PMC5734749
- DOI: 10.1371/journal.pone.0189536
Hypertonic saline attenuates the cytokine-induced pro-inflammatory signature in primary human lung epithelia
Abstract
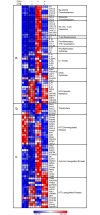
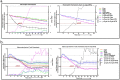
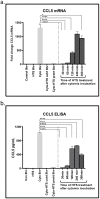
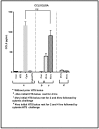
Trauma/hemorrhagic shock is a complex physiological phenomenon that leads to dysregulation of many molecular pathways. For over a decade, hypertonic saline (HTS) has been used as an alternative resuscitation fluid in the setting of trauma/hemorrhagic shock. In addition to restoring circulating volume within the vascular space, studies have shown a positive immunomodulatory effect of HTS. Targeted studies have shown that HTS affects the transcription of several pro-inflammatory cytokines by inhibiting the NF-κB-IκB pathway in model cell lines and rats. However, few studies have been undertaken to assess the unbiased effects of HTS on the whole transcriptome. This study was designed to interrogate the global transcriptional responses induced by HTS and provides insight into the underlying molecular mechanisms and pathways affected by HTS. In this study, RNA sequencing was employed to explore early changes in transcriptional response, identify key mediators, signaling pathways, and transcriptional modules that are affected by HTS in the presence of a strong inflammatory stimulus. Our results suggest that primary human small airway lung epithelial cells (SAECS) exposed to HTS in the presence and absence of a strong pro-inflammatory stimulus exhibit very distinct effects on cellular response, where HTS is highly effective in attenuating cytokine-induced pro-inflammatory responses via mechanisms that involve transcriptional regulation of inflammation which is cell type and stimulus specific. HTS is a highly effective anti-inflammatory agent that inhibits the chemotaxis of leucocytes towards a pro-inflammatory gradient and may attenuate the progression of both the innate and adaptive immune response.
Conflict of interest statement
Figures


Similar articles
-
Hypertonic Saline Primes Activation of the p53-p21 Signaling Axis in Human Small Airway Epithelial Cells That Prevents Inflammation Induced by Pro-inflammatory Cytokines.J Proteome Res. 2016 Oct 7;15(10):3813-3826. doi: 10.1021/acs.jproteome.6b00602. Epub 2016 Aug 29. J Proteome Res. 2016. PMID: 27529569 Free PMC article.
-
Hypertonic saline resuscitation from hemorrhagic shock does not impair the neutrophil response to intraabdominal infection.Surgery. 2008 Nov;144(5):814-21. doi: 10.1016/j.surg.2008.07.008. Epub 2008 Sep 14. Surgery. 2008. PMID: 19081025
-
Hypertonic resuscitation of hemorrhagic shock upregulates the anti-inflammatory response by alveolar macrophages.Surgery. 2003 Aug;134(2):312-8. doi: 10.1067/msy.2003.246. Surgery. 2003. PMID: 12947335
-
Innate immunity and inflammation: a transcriptional paradigm.Immunol Res. 2001;23(2-3):99-109. doi: 10.1385/IR:23:2-3:099. Immunol Res. 2001. PMID: 11444396 Review.
-
Hypertonic saline and the microcirculation.J Trauma. 2003 May;54(5 Suppl):S133-40. doi: 10.1097/01.TA.0000064526.33647.63. J Trauma. 2003. PMID: 12768115 Review.
Cited by
-
Neutrophils in the initiation and resolution of acute pulmonary inflammation: understanding biological function and therapeutic potential.J Pathol. 2019 Apr;247(5):672-685. doi: 10.1002/path.5221. Epub 2019 Feb 15. J Pathol. 2019. PMID: 30570146 Free PMC article. Review.
-
Effects of Hypertonic Saline and Hydroxyethyl Starch on Myeloid-Derived Suppressor Cells in Hemorrhagic Shock Mice under Secondary Bacterial Attack.Biomed Res Int. 2020 Mar 9;2020:5417201. doi: 10.1155/2020/5417201. eCollection 2020. Biomed Res Int. 2020. PMID: 32258126 Free PMC article.
-
Hypotheses about sub-optimal hydration in the weeks before coronavirus disease (COVID-19) as a risk factor for dying from COVID-19.Med Hypotheses. 2020 Nov;144:110237. doi: 10.1016/j.mehy.2020.110237. Epub 2020 Sep 2. Med Hypotheses. 2020. PMID: 33254543 Free PMC article.
-
Inflammatory signature in lung tissues in patients with combined pulmonary fibrosis and emphysema.Biomarkers. 2019 May;24(3):232-239. doi: 10.1080/1354750X.2018.1542458. Epub 2018 Nov 19. Biomarkers. 2019. PMID: 30411980 Free PMC article.
-
Quantitative Investigation into the influence of intravenous fluids on human immune and cancer cell lines.Sci Rep. 2020 Jul 16;10(1):11792. doi: 10.1038/s41598-020-61296-5. Sci Rep. 2020. PMID: 32678120 Free PMC article.
References
-
- Tisherman SA, Schmicker RH, Brasel KJ, Bulger EM, Kerby JD, Minei JP, et al. Detailed Description of all Deaths in Both the Shock and Traumatic Brain Injury Hypertonic Saline Trials of the Resuscitation Outcomes Consortium. Ann Surg. 2015;261(3):586–90. 10.1097/SLA.0000000000000837 - DOI - PMC - PubMed
-
- Sauaia A, Moore EE, Johnson JL, Chin TL, Banerjee A, Sperry JL, et al. Temporal trends of postinjury multiple-organ failure: still resource intensive, morbid, and lethal. The journal of trauma and acute care surgery. 2014;76(3):582–92, discussion 92–3. Epub 2014/02/21. 10.1097/TA.0000000000000147 - DOI - PMC - PubMed
-
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–82. http://www.nature.com/nri/journal/v6/n3/suppinfo/nri1785_S1.html. - PubMed
-
- Weigand MA, Horner C, Bardenheuer HJ, Bouchon A. The systemic inflammatory response syndrome. Best Pract Res Clin Anaesthesiol. 2004;18(3):455–75. Epub 2004/06/24. . - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

