Bacteriophage DNA glucosylation impairs target DNA binding by type I and II but not by type V CRISPR-Cas effector complexes
- PMID: 29253268
- PMCID: PMC5778469
- DOI: 10.1093/nar/gkx1264
Bacteriophage DNA glucosylation impairs target DNA binding by type I and II but not by type V CRISPR-Cas effector complexes
Abstract
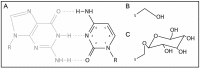
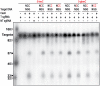
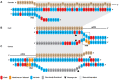
Prokaryotes encode various host defense systems that provide protection against mobile genetic elements. Restriction-modification (R-M) and CRISPR-Cas systems mediate host defense by sequence specific targeting of invasive DNA. T-even bacteriophages employ covalent modifications of nucleobases to avoid binding and therefore cleavage of their DNA by restriction endonucleases. Here, we describe that DNA glucosylation of bacteriophage genomes affects interference of some but not all CRISPR-Cas systems. We show that glucosyl modification of 5-hydroxymethylated cytosines in the DNA of bacteriophage T4 interferes with type I-E and type II-A CRISPR-Cas systems by lowering the affinity of the Cascade and Cas9-crRNA complexes for their target DNA. On the contrary, the type V-A nuclease Cas12a (also known as Cpf1) is not impaired in binding and cleavage of glucosylated target DNA, likely due to a more open structural architecture of the protein. Our results suggest that CRISPR-Cas systems have contributed to the selective pressure on phages to develop more generic solutions to escape sequence specific host defense systems.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
-
- Wigington C.H., Sonderegger D., Brussaard C.P.D., Buchan A., Finke J.F., Fuhrman J.A., Lennon J.T., Middelboe M., Suttle C.A., Stock C. et al. Re-examination of the relationship between marine virus and microbial cell abundances. Nat. Microbiol. 2016; 1:15024. - PubMed
-
- Samson J.E., Magadán A.H., Sabri M., Moineau S.. Revenge of the phages: defeating bacterial defences. Nat. Rev. Microbiol. 2013; 11:675–687. - PubMed
-
- Sain B., Murray N.E.. The hsd (host specificity) genes of E. coli K 12. Mol. Gen. Genet. 1980; 180:35–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

