Bisphosphonates in multiple myeloma: an updated network meta-analysis
- PMID: 29253322
- PMCID: PMC6486151
- DOI: 10.1002/14651858.CD003188.pub4
Bisphosphonates in multiple myeloma: an updated network meta-analysis
Abstract
Background: Bisphosphonates are specific inhibitors of osteoclastic activity and are used in the treatment of patients with multiple myeloma (MM). While bisphosphonates are shown to be effective in reducing vertebral fractures and pain, their role in improving overall survival (OS) remains unclear. This is an update of a Cochrane review first published in 2002 and previously updated in 2010 and 2012.
Objectives: To assess the evidence related to benefits and harms associated with use of various types of bisphosphonates (aminobisphosphonates versus non-aminobisphosphonates) in the management of patients with MM. Our primary objective was to determine whether adding bisphosphonates to standard therapy in MM improves OS and progression-free survival (PFS), and decreases skeletal-related morbidity. Our secondary objectives were to determine the effects of bisphosphonates on pain, quality of life, incidence of hypercalcemia, incidence of bisphosphonate-related gastrointestinal toxicities, osteonecrosis of jaw (ONJ) and hypocalcemia.
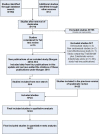
Search methods: We searched MEDLINE, Embase (September 2011 to July 2017) and the CENTRAL (2017, Issue 7) to identify all randomized controlled trial (RCT) in MM up to July 2017 using a combination of text and MeSH terms.
Selection criteria: Any randomized controlled trial (RCT) comparing bisphosphonates versus placebo/no treatment/bisphosphonates and observational studies or case reports examining bisphosphonate-related ONJ in patients with MM were eligible for inclusion.
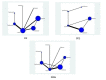
Data collection and analysis: Two review authors extracted the data. Data were pooled and reported as hazard ratio (HR) or risk ratio (RR) using a random-effects model. We used meta-regression to explore statistical heterogeneity. Network meta-analysis using Bayesian approach was conducted.
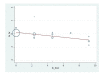
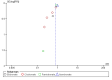
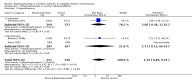
Main results: In this update, we included four new studies (601 participants), resulting in a total of 24 included studies.Twenty RCTs compared bisphosphonates with either placebo or no treatment and four RCTs involved another bisphosphonate as a comparator. The 24 included RCTs enrolled 7293 participants. Pooled results showed that there was moderate-quality evidence of a reduction in mortality with on OS from 41% to 31%, but the confidence interval is consistent with a larger reduction and small increase in mortality compared with placebo or no treatment (HR 0.90, 95% CI 0.76 to 1.07; 14 studies; 2706 participants). There was substantial heterogeneity among the included RCTs (I2 = 65%) for OS. To explain this heterogeneity we performed a meta-regression assessing the relationship between bisphosphonate potency and improvement in OS, which found an OS benefit with zoledronate but limited evidence of an effect on PFS. This provided a further rationale for performing a network meta-analyses of the various types of bisphosphonates that were not compared head-to-head in RCTs. Results from network meta-analyses showed evidence of a benefit for OS with zoledronate compared with etidronate (HR 0.56, 95% CI 0.29 to 0.87) and placebo (HR 0.67, 95% CI 0.46 to 0.91). However, there was no evidence for a difference between zoledronate and other bisphosphonates.The effect of bisphosphonates on disease progression (PFS) is uncertain. Based on the HR of 0.75 (95% CI 0.57 to 1.00; seven studies; 908 participants), 47% participants would experience disease progression without treatment compared with between 30% and 47% with bisphosphonates (low-quality evidence). There is probably a similar risk of non-vertebral fractures between treatment groups (RR 1.03, 95% CI 0.68 to 1.56; six studies; 1389 participants; moderate-quality evidence). Pooled analysis demonstrated evidence for a difference favoring bisphosphonates compared with placebo or no treatment on prevention of pathological vertebral fractures (RR 0.74, 95% CI 0.62 to 0.89; seven studies; 1116 participants; moderate-quality evidence) and skeletal-related events (SREs) (RR 0.74, 95% CI 0.63 to 0.88; 10 studies; 2141 participants; moderate-quality evidence). The evidence for less pain with bisphosphonates was of very low quality (RR 0.75, 95% CI 0.60 to 0.95; eight studies; 1281 participants).Bisphosphonates may increase ONJ compared with placebo but the confidence interval is very wide (RR 4.61, 95% CI 0.99 to 21.35; P = 0.05; six studies; 1284 participants; low-quality evidence). The results from the network meta-analysis did not show any evidence for a difference in the incidence of ONJ (eight RCTs, 3746 participants) between bisphosphonates. Data from nine observational studies (1400 participants) reported an incidence of 5% to 51% with combination of pamidronate and zoledronate, 3% to 11% with zoledronate alone, and 0% to 18% with pamidronate alone.The pooled results showed no evidence for a difference in increase in frequency of gastrointestinal symptoms with the use of bisphosphonates compared with placebo or no treatment (RR 1.23, 95% CI 0.95 to 1.59; seven studies; 1829 participants; low-quality evidence).The pooled results showed no evidence for a difference in increase in frequency of hypocalcemia with the use of bisphosphonates compared with placebo or no treatment (RR 2.19, 95% CI 0.49 to 9.74; three studies; 1090 participants; low-quality evidence). The results from network meta-analysis did not show any evidence for differences in the incidence of hypocalcemia, renal dysfunction and gastrointestinal toxicity between the bisphosphonates used.
Authors' conclusions: Use of bisphosphonates in participants with MM reduces pathological vertebral fractures, SREs and pain. Bisphosphonates were associated with an increased risk of developing ONJ. For every 1000 participants treated with bisphosphonates, about one patient will suffer from the ONJ. We found no evidence of superiority of any specific aminobisphosphonate (zoledronate, pamidronate or ibandronate) or non-aminobisphosphonate (etidronate or clodronate) for any outcome. However, zoledronate was found to be better than placebo and first-generation bisposphonate (etidronate) in pooled direct and indirect analyses for improving OS and other outcomes such as vertebral fractures. Direct head-to-head trials of the second-generation bisphosphonates are needed to settle the issue if zoledronate is truly the most efficacious bisphosphonate currently used in practice.
Conflict of interest statement
RM: None known.
AK: None known.
BM: None known.
BD: None known.
Figures





















Update of
-
Bisphosphonates in multiple myeloma: a network meta-analysis.Cochrane Database Syst Rev. 2012 May 16;(5):CD003188. doi: 10.1002/14651858.CD003188.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2017 Dec 18;12:CD003188. doi: 10.1002/14651858.CD003188.pub4. PMID: 22592688 Updated.
References
References to studies included in this review
Attal 2006 {published data only}
-
- Attal M, Harousseau JL, Leyvraz S, Doyen C, Hulin C, Benboubker L, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood 2006;108(10):3289‐94. [PUBMED: 16873668] - PubMed
Aviles 2007 {published data only}
-
- Aviles A, Nambo MJ, Neri N, Castaneda C, Cleto S, Huerta‐Guzman J. Antitumor effect of zoledronic acid in previously untreated patients with multiple myeloma. Medical Oncology (Northwood, London, England) 2007;24(2):227‐30. [PUBMED: 17848748] - PubMed
Aviles 2013 {published data only}
-
- Aviles A, Neri N, Huerta‐Guzman J, Nambo MJ. Randomized clinical trial of zoledronic acid in multiple myeloma patients undergoing high‐dose chemotherapy and stem‐cell transplantation. Current oncology (Toronto, Ont.) 2013; Vol. 20, issue 1:e13‐20. [DOI: 10.3747/co.20.1055; CN‐00912202] - DOI - PMC - PubMed
Belch 1991 {published data only}
-
- Belch AR, Bergsagel DE, Wilson K. Effect of daily etidronate on the osteolysis of multiple myeloma. Journal of Clinical Oncology 1991;9:1397‐402. [MEDLINE: ] - PubMed
Berenson 1998a {published data only}
-
- Berenson JR, Lichtenstein A, Porter L. Long‐term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Journal of Clinical Oncology 1998;16:593‐602. [MEDLINE: ] - PubMed
-
- Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. New England Journal of Medicine 1996;334:488‐93. - PubMed
Brincker 1998 {published data only}
-
- Abildgaard N, Rungby J, Glerup H, Brixen K, Kassem M, Brincker H, et al. Long‐term oral pamidronate treatment inhibits osteoclastic bone resorption and bone turnover without affecting osteoblastic function in multiple myeloma. European Journal of Hematology 1998;61:128‐34. - PubMed
-
- Brincker JW, Abildgaard N. Failure of oral pamidronate to reduce skeletal morbidity in multiple myeloma: a double‐blind placebo‐controlled trial. British Journal of Haematology 1998;101:280‐6. [MEDLINE: ] - PubMed
Daragon 1993 {published data only}
-
- Daragon A, Humez C, Michot CXLL. Treatment of multiple myeloma with etidronate results of a multicentre double‐blind study. European Journal of Medicine 1993;2:449‐52. [MEDLINE: ] - PubMed
Delmas 1982 {published data only}
-
- Delmas PD, Charhon S, Chapuy MC, Vignon E, Briancon D, Edouard C, et al. Long‐term effects of dichloromethylene diphosphonate (CI2MDP) on skeletal lesions in multiple myeloma. Metabolic Bone Disease and Related Research 1982;4:163‐8. [MEDLINE: ] - PubMed
Garcia‐Sanz 2015 {published data only}
Gimsing 2010 {published data only}
-
- Gimsing P, Carlson K, Turesson I, Fayers P, Waage A, Vangsted A, et al. Effect of pamidronate 30 mg versus 90 mg on physical function in patients with newly diagnosed multiple myeloma (Nordic Myeloma Study Group): a double‐blind, randomised controlled trial. Lancet Oncology 2010;11(10):973‐82. [PUBMED: 20863761] - PubMed
Heim 1995 {published and unpublished data}
-
- Clemens MR, Fessele K, Heim ME. Multiple myeloma: effect of daily dichloromethylene bisphosphonate on skeletal Multiple myeloma: effect of daily dichloromethylene bisphosphonate on skeletal complications. Annals of Hematology 1993;66:141‐6. - PubMed
-
- Heim ME, Clemens MR, Queißer W, Pecherstorfer M, Boewer C, Herold M, et al. Prospective randomized trial of dichloromethilene bisphosphonate (clodronate) in patients with multiple myeloma. Onkologie 1995;18:439‐48.
Kraj 2000 {published data only}
-
- Kraj M, Poglód R, Pawlikowsky J, Maj S. The effect of long‐term pamidronate treatment on skeletal morbidity in advanced multiple myeloma. Acta Haematologica Polonica 2000;31:379‐89.
-
- Kraj M, Póglod R, Pawlikowski J, Maj S, Nasiloska B. Effect of pamidronate on skeletal morbidity in myelomatosis. Part 1: The results of the 12 months of pamidronate therapy. Acta Poloniae Pharmaceutica 2000;57 (suppl 1):113‐6. - PubMed
Lahtinen 1992 {published data only}
-
- Lahtinen R, Laakso M, Palva I. Randomized, placebo‐controlled multicentre trial of clodronate in multiple myeloma. Lancet 1992;340:1049‐52. [MEDLINE: ] - PubMed
Leng 2002 {published data only}
-
- Leng Y, Chen SL, Shi HZ. Effects of pamidronate disodium (Bonin) combined with chemotherapy on bone pain in multiple myeloma. Hang tian yi xue yu yi xue gong cheng [Space Medicine & Medical Engineering] 2002;15(5):377‐8. [PUBMED: 12449148] - PubMed
McCloskey 2001 {published and unpublished data}
-
- McCloskey EV, Dunn JA, Kanis JA, MacLennan ICM, Drayson MT. Long‐term follow‐up of a prospective, double‐blind, placebo‐controlled randomised trial of clodronate in multiple myeloma. British Journal of Haematology 2001;113:1035‐43. [MEDLINE: ] - PubMed
-
- McCloskey EV, MacLennan IC, Drayson MT, Chapman C, Dunn J, Kanis JA. A randomized trial of the effect of clodronate on skeletal morbidity in multiple myeloma. MRC Working Party on Leukaemia in Adults. British Journal of Haematology 1998;100:317‐25. - PubMed
Menssen 2002 {published data only}
-
- Fontana A, Herrmann Z, Menssen HD, Sakalova A, Boewer C, Facon T, et al. Effects of intravenous ibandronate therapy on skeletal related events (SRE) and survival in patients with advanced multiple myeloma. Blood 1998;92(Suppl 1):106a. - PubMed
-
- Menssen HD, Sakalová A, Fontana A, Herrmann Z, Boewer C, Facon T, et al. Effects of long‐term intravenous ibandronate therapy on skeletal‐related events, survival and bone resorption in patients with advanced multiple myeloma. Journal of Clinical Oncology 2002;20 (9):2353‐9. - PubMed
Morgan 2010 {published data only}
-
- Jackson GH, Morgan GJ, Davies FE, Wu P, Gregory WM, Bell SE, et al. Osteonecrosis of the jaw and renal safety in patients with newly diagnosed multiple myeloma: Medical Research Council Myeloma IX Study results. British Journal of Haematology 2014;166(1):109‐17. [PUBMED: 24673708] - PubMed
-
- Larocca A, Child JA, Cook G, Jackson GH, Russell N, Szubert A, et al. The impact of response on bone‐directed therapy in patients with multiple myeloma. Blood 2013;122(17):2974‐7. [PUBMED: 23974194] - PubMed
-
- Morgan GJ, Davies FE, Gregory WM, Bell SE, Szubert AJ, Cook G, et al. Long‐term follow‐up of MRC Myeloma IX trial: Survival outcomes with bisphosphonate and thalidomide treatment. Clinical Cancer Research : an official journal of the American Association for Cancer Research 2013;19(21):6030‐8. [PUBMED: 23995858] - PubMed
-
- Morgan GJ, Jackson GH, Davies F, Wu P, Gregory W, Bell SE, et al. Efficacy and side‐effect profile of long‐term bisphosphonate therapy in patients (pts) with multiple myeloma (MM): MRC myeloma IX study results. Journal of Clinical Oncology 2012; Vol. 30, issue 15 SUPPL. 1. [CN‐01028214]
Musto 2003 {published data only}
-
- D'arena G, Gobbi PG, Broglia C, Sacchi S, Quarta G, Baldini L, et al. Pamidronate versus observation in asymptomatic myeloma: final results with long‐term follow‐up of a randomized study. Leukemia & Lymphoma 2011;1:1‐5. [PUBMED: 21299465] - PubMed
-
- Musto P, Falcone A, Sanpaolo G, Bodenizza C, Cascavilla N, Melillo L, et al. Pamidronate reduces skeletal events but does not improve progression‐free survival in early‐stage untreated myeloma: results of a randomized trial. Leukemia & Lymphoma 2003;44(9):1545‐8. [PUBMED: 14565658] - PubMed
Musto 2008 {published data only}
-
- Musto P, Petrucci MT, Bringhen S, Guglielmelli T, Caravita T, Bongarzoni V, et al. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer 2008;113(7):1588‐95. [PUBMED: 18683218] - PubMed
Rosen 2003 {published data only}
-
- Berenson JR, Rosen LS, Howell A, Porter L, Coleman RE, Morley W, et al. Zoledronic acid reduces skeletal‐related events in patients with osteolytic metastases: a double‐blind, randomized dose‐response study. Cancer 2001;91(7):1191‐200. - PubMed
-
- Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, et al. Long term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma. Cancer 2003;98(8):1735‐44. - PubMed
-
- Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double‐blind, comparative trial. Cancer 2001;7(5):377‐87. - PubMed
-
- Rosen LS, Gordon DH, Dugan W Jr, Major P, Eisenberg PD, Provencher L, et al. Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer 2004;100:36‐43. - PubMed
Sezer 2010 {published data only}
-
- Sezer O, Jakob C, Aldaoud A, Schmidt K, Schwarzer A, Maintz C, et al. Zoledronic acid therapy versus control in patients with multiple myeloma in stage I (Durie & Salmon): results of a phase III study of the DSMM and OSHO. 15th Congress of the European Hematology Association Abstr 0361. Barcelona, Spain, Jun 10–13, 2010.
Terpos 2000 {published and unpublished data}
-
- Terpos E, Palermos J, Tsionos K, Anargyrou K, Viniou N, Papassavas P, et al. Effect of pamidronate administration on markers of bone turnover and disease activity in multiple myeloma. European Journal of Haematology 2000;65:331‐6. - PubMed
Terpos 2003 {published data only (unpublished sought but not used)}
-
- Terpos E, Viniou N, Fuente J, Meletis J, Voskaridou E, Karkantaris C, et al. Pamidronate is superior to ibandronate in decreasing bone resorption, interleukin‐6and b2‐microglobulin in multiple myeloma. European Journal of Haematology 2003;70:34‐42. - PubMed
Zhang 2012 {published data only}
-
- Zhang X, Chang C, Zhao Y, Wu L, Zhang Z, Li X. The effect of the combination of bisphosphonates and conventional chemotherapy on bone metabolic markers in multiple myeloma patients. Hematology (Amsterdam, Netherlands) 2012;17(5):255‐60. [PUBMED: 22971530] - PubMed
-
- Zhang X, Chang CK, Zhang Z, Zhao YS, Xiao C, Li X. [Influence of bisphosphonate combined with chemotherapy on bone mineral density of patients with multiple myeloma]. Zhongguo shi yan xue ye xue za zhi / Zhongguo bing li sheng li xue hui [Journal of experimental hematology / Chinese Association of Pathophysiology] 2012;20(5):1135‐8. [PUBMED: 23114134] - PubMed
References to studies excluded from this review
Ali 2001 {published data only}
-
- Ali SM, Esteva FJ, Hortobagyi G, Harvey H, Seaman J, Knight R, et al. Safety and efficacy of bisphosphonates beyond 24 months in cancer patients. Journal of Clinical Oncology 2001;19(14):3434‐7. - PubMed
Barlogie 2008 {published data only}
Bergner 2007 {published data only}
-
- Bergner R, Henrich DM, Hoffmann M, Honecker A, Mikus G, Nauth B, et al. Renal safety and pharmacokinetics of ibandronate in multiple myeloma patients with or without impaired renal function. Journal of Clinical Pharmacology 2007;47(8):942‐50. - PubMed
Caparrotti 2003 {published data only}
-
- Caparrotti G, Catalano L, Feo C, Vallone R, Pagnini D, Rotoli B. Perspective study on pamidronate in stage I multiple myeloma. Hematology Journal 2003;4(6):459‐60. - PubMed
Chiang 2013 {published data only}
-
- Chiang PH, Wang HC, Lai YL, Chen SC, Yen‐Hwa W, Kok CK, et al. Zoledronic acid treatment for cancerous bone metastases: a phase IV study in Taiwan. Journal of Cancer Research and Therapeutics 2013;9(4):653‐9. [PUBMED: 24518712] - PubMed
Ciepluch 2002 {published data only}
-
- Ciepluch H, Baran W, Hellmann A. Combination of pamidronate and thalidomide in the therapy of treatment‐resistant multiple myeloma. Medical Science Monitor 2002;8(4):PI31‐PI36. - PubMed
Delea 2012 {published data only}
-
- Delea TE, Rotter J, Taylor M, Chandiwana D, Bains M, Ouagari K, et al. Cost‐effectiveness of zoledronic acid vs clodronic acid for newly‐diagnosed multiple myeloma from the United Kingdom healthcare system perspective. Journal of Medical Economics 2012;15(3):454‐64. [PUBMED: 22316275] - PubMed
Fizazi 2009 {published data only}
-
- Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. Journal of Clinical Oncology 2009;27(10):1564‐71. [PUBMED: 19237632] - PubMed
Henry 2014 {published data only}
-
- Henry D, Vadhan‐Raj S, Hirsh V, Moos R, Hungria V, Costa L, et al. Delaying skeletal‐related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Supportive Care in Cancer : official journal of the Multinational Association of Supportive Care in Cancer 2014;22(3):679‐87. [PUBMED: 24162260] - PubMed
Kraj 2000a {published data only}
-
- Kraj M, Póglod R, Pawlikowski J, Maj S, Nasilowska B. Effect of pamidronate on skeletal morbidity in myelomatosis. Part 1. The results of the first 12 months of pamidronate therapy. Acta Poloniae Pharmaceutica 2000;57(suppl 1):113‐6. - PubMed
Kraj 2002 {published data only}
-
- Kraj M, Poglod R, Maj S, Pawlikowski J, Sokolowska U, Szczepanik J. Comparative evaluation of safety and efficacy of pamidronate and zoledronic acid in multiple myeloma patients (single center experience). Acta Poloniae Pharmaceutica 2002;59(6):478‐82. [PUBMED: 12669777] - PubMed
Lipton 2012 {published data only}
-
- Lipton A, Fizazi K, Stopeck AT, Henry DH, Brown JE, Yardley DA, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal‐related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. European Journal of Cancer (Oxford, England : 1990) 2012;48(16):3082‐92. [PUBMED: 22975218] - PubMed
Martin 2002 {published data only}
-
- Martín A, García‐Sanz R, Hernández J, Bladé J, Suquía B, Fernández‐Calvo J, et al. Pamidronate induces bone formation in patients with smouldering or indolent myeloma, with no significant anti‐tumour effect. British Journal of Haematology 2002;118(1):239‐42. - PubMed
Morris 2001 {published data only}
-
- Morris TC, Ranaghan L, Morrison J. Phase II trial of clarithromycin and pamidronate therapy in myeloma. Medical Oncology 2001;18(1):79‐84. - PubMed
Spencer 2008 {published data only}
Tassinari 2007 {published data only}
-
- Tassinari D, Poggi B, Nicoletti S, Fantini M, Tamburini E, Possenti C, et al. Zoledronic acid treatment at home: safety data from an observational prospective trial. Journal of Palliative Medicine 2007;10(2):352‐8. - PubMed
Teoh 2012 {published data only}
-
- Teoh G, Chen Y, Kim K, Srivastava A, Pai VR, Yoon SS, et al. Lower dose dexamethasone/thalidomide and zoledronic acid every 3 weeks in previously untreated multiple myeloma. Clinical Lymphoma, Myeloma & Leukemia 2012;12(2):118‐26. [PUBMED: 22206804] - PubMed
Terpos 2010 {published data only}
-
- Terpos E, Berenson J, Cook RJ, Lipton A, Coleman RE. Prognostic variables for survival and skeletal complications in patients with multiple myeloma osteolytic bone disease. Leukemia 2010;24(5):1043‐9. [PUBMED: 20376081] - PubMed
Tosi 2006a {published data only}
-
- Tosi P, Zamagni E, Cellini C, Parente R, Cangini D, Tacchetti P, et al. First‐line therapy with thalidomide, dexamethasone and zoledronic acid decreases bone resorption markers in patients with multiple myeloma. European Journal of Haematology 2006;76(5):399‐404. - PubMed
Vadhan‐Raj 2012 {published data only}
-
- Vadhan‐Raj S, Moos R, Fallowfield LJ, Patrick DL, Goldwasser F, Cleeland CS, et al. Clinical benefit in patients with metastatic bone disease: results of a phase 3 study of denosumab versus zoledronic acid. Annals of Oncology : official journal of the European Society for Medical Oncology / ESMO 2012;23(12):3045‐51. [PUBMED: 22851406] - PubMed
Vij 2009 {published data only}
-
- Vij R, Horvath N, Spencer A, Taylor K, Vadhan‐Raj S, Vescio R, et al. An open‐label, phase 2 trial of denosumab in the treatment of relapsed or plateau‐phase multiple myeloma. American Journal of Hematology 2009;84(10):650‐6. [PUBMED: 19714603] - PubMed
Vogel 2004 {published data only}
-
- Vogel CL, Yanagihara RH, Wood AJ, Schnell FM, Henderson C, Kaplan BH, et al. Safety and pain palliation of zoledronic acid in patients with breast cancer, prostate cancer, or multiple myeloma who previously received bisphosphonate therapy. Oncologist 2004;9(6):687‐95. - PubMed
Witzig 2013 {published data only}
-
- Witzig TE, Mandrekar S, Detweiler‐Short K, Q Lacy M, Laumann K, Dispenzieri A, et al. A phase III randomized trial of thalidomide (THAL) plus zoledronic acid (ZLD) versus zoledronic acid alone in patients with early stage multiple myeloma (MC0289). Blood 2010; Vol. 116, issue 21. [CN‐01032934]
References to ongoing studies
Lund 2014 {published data only}
-
- Thomas Lund. Magnolia study prolonged protection from bone disease in multiple myeloma. An open label phase 3 multicenter international randomised trial. https://clinicaltrials.gov/ct2/show/NCT02286830.
Additional references
Acito 1994
-
- Acito AJ, Kasra M, Lee JM, Grynpas MD. Effect of intermittent administration of pamidronate on the mechanical proprieties of canine cortical and trabecular bone. Journal of Orthopedic Research 1994;12:742‐6. - PubMed
Alexanian 1994
-
- Alexanian R, Dimopulos M. The treatment of multiple myeloma. New England Journal of Medicine 1994;330:484‐9. - PubMed
Anderson 2015
Aparicio 1998
-
- Aparicio A, Gardner A, Tu Y, Savage A, Berenson J, Lichtenstein A. In vitro cytoreductive effects on multiple myeloma cells induced by bisphosphonates. Leukemia 1998;12:220‐9. - PubMed
Atula 2003
-
- Atula S, Powles T, Paterson A, McCloskey E, Nevalainen J, Kanis J. Extended safety profile of oral clodronate after long term use in primary breast cancer patients. Drug Safety 2003;26:661‐71. - PubMed
Badros 2006
-
- Badros A, Weikel D, Salama A, Goloubeva O, Schneider A, Rapoport A, et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. Journal of Clinical Oncology 2006;24(6):945‐52. - PubMed
Bagan 2006
-
- Bagan JV, Jimenez Y, Murillo J, Hernandez S, Poveda R, Sanchis JM, et al. Jaw osteonecrosis associated with bisphosphonates: multiple exposed areas and its relationship to teeth extractions. Study of 20 cases [Letter]. Oral Oncology 2006;42:327‐9. - PubMed
Balshem 2011
-
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines 3: Rating the quality of evidence ‐ introduction. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] - PubMed
Battley 2006
-
- Battley J, Jayathissa S, Seneviratne E. Jaw osteonecrosis associated with bisphosphonates. New Zealand Medical Journal 2006;119(1246):U2341. - PubMed
Begg 1994
-
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PUBMED: 7786990] - PubMed
Berenson 1998b
-
- Berenson JR. The efficacy of pamidronate disodium in the treatment of osteolytic lesions and bone pain in multiple myeloma. Reviews in Contemporary Pharmacotherapy 1998;9:195‐203.
Berenson 2011
-
- Berenson JR, Yellin O, Crowley J, Makary A, Gravenor DS, Yang HH, et al. Prognostic factors and jaw and renal complications among multiple myeloma patients treated with zoledronic acid. American Journal of Hematology 2011;86(1):25‐30. [PUBMED: 21120861] - PubMed
Braun 2006
-
- Braun E, Iacono VJ. Bisphosphonates: case report of non surgical periodontal therapy and osteochemonecrosis. International Journal of Periodontics and Restorative Dentistry 2006;26(4):315‐9. - PubMed
Broglia 2006
-
- Broglia C, Merlati G, Valentino F, Benatti C, Gobbi P, Ascari E, et al. Avascular jaw osteonecrosis associated with bisphosphonate therapy. Recenti Progressi in Medicina (Roma) 2006;97(3):140‐4. - PubMed
Brown 2004
-
- Brown JE, Neville‐Webbe H, Coleman RE. The role of bisphosphonates in breast and prostate cancers. Endocrine Related Cancer 2004;11:207‐24. - PubMed
Bucher 1997
-
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [PUBMED: 9250266] - PubMed
Bujanda 2007
-
- Bujanda AD, Sarmiento BU, Suarez CMA, Morales AJ. Assessment of renal toxicity and osteonecrosis of the jaws in patients receiving zoledronic acid for bone metastasis. Annals of Oncology 2007;18(3):556‐60. - PubMed
Calvo‐Villas 2006
-
- Calvo‐Villas JM, Tapia Torres M, Govantes Rodríguez J, Carreter de Granda E, Sicilia Guillén F. Osteonecrosis of the jaw in patients with multiple myeloma during and after treatment with zoledronic acid. Medicina Clinicia (Barcelona) 2006;127(15):576‐9. - PubMed
Capalbo 2006
-
- Capalbo S, Delia M, Diomede D, Dargenio M, Chiefa A, Favia G, et al. Jaw osteonecrosis associated with use of bisphosphonates and chemotherapy: paradoxical complication of treatment of bone lesions in multiple myeloma patients. International Journal of Hematology 2006;83(5):439‐42. - PubMed
Carneiro 2006
Carter 2005
-
- Carter G, Goss AN, Doecke C. Bisphosphonates and avascular necrosis of the jaw: a possible association. Medical Journal of Australia (Sydney) 2005;182:413‐5. - PubMed
Cassidy 2006
-
- Cassidy J, Bisset D, Spence RAJ, Payne M, editors. Oxford Handbook of Oncology. New York: Oxford University Press, 2006:504‐5.
Cetiner 2009
-
- Cetiner S, Sucak GT, Kahraman SA, Aki SZ, Kocakahyaoglu B, Gultekin SE, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with zoledronic acid. Journal of Bone and Mineral Metabolism 2009;27(4):435‐43. [PUBMED: 19240969] - PubMed
Chaimani 2013
Chang 2003
-
- Chang JT, Green L, Beitz J. Renal failure with the use of zoledronic acid. New England Journal of Medicine 2003;349:1676‐9. - PubMed
Clarke 2007
-
- Clarke BM, Boyette J, Vural E, Suen JY, Anaissie EJ, Stack BC Jr. Bisphosphonates and jaw osteonecrosis: the UAMS experience. Otolaryngology‐‐Head and Neck Surgery 2007;136(3):396‐400. - PubMed
Cornell 2015
-
- Cornell JE. The PRISMA extension for network meta‐analysis: bringing clarity and guidance to the reporting of systematic reviews incorporating network meta‐analyses. Annals of Internal Medicine 2015; Vol. 162, issue 11:797‐8. [PUBMED: 26030637] - PubMed
Corso 2007
-
- Corso A, Varettoni M, Zappasodi P, Klersy C, Mangiacavalli S, Pica G, et al. A different schedule of zoledronic acid can reduce the risk of the osteonecrosis of the jaw in patients with multiple myeloma. Leukemia 2007;21(7):1545‐8. - PubMed
Curi 2007
-
- Curi MM, Cossolin GS, Koga DH, Araújo SR, Feher O, dos Santos MO, et al. Treatment of avascular osteonecrosis of the mandible in cancer patients with a history of bisphosphonate therapy by combining bone resection and autologous platelet‐rich plasma: report of 3 cases. Journal of Oral Maxillofacial Surgery 2007;65(2):349‐55. - PubMed
Dannemann 2007
-
- Dannemann C, Graetz KW, Riener MO, Zwahlen RA. Jaw osteonecrosis related to bisphosphonate therapy: a severe secondary disorder. Bone 2007;40(4):828‐34. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [PUBMED: 3802833] - PubMed
Dhodapkar 1998
-
- Dhodapkar MV, Singh J, Mehta J, Fassas A, Desikan KR, Perlman M, et al. Anti‐myeloma activity of pamidronate in vivo. British Journal of Haematology 1998;103:530‐2. - PubMed
Dias 2010
-
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29(7‐8):932‐44. [PUBMED: 20213715] - PubMed
Diego 2007
-
- Diego R, D'Orto O, Pagani D, Agazzi A, Marzano U, Derada Troletti G, et al. Bisphosphonate‐associated osteonecrosis of the jaws: a therapeutic dilemma. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2007;103(3):e1‐5. - PubMed
Dimitrakopoulos 2006
-
- Dimitrakopoulos I, Magopoulos C, Karakasis D. Bisphosphonate‐induced avascular osteonecrosis of the jaws: a clinical report of 11 cases. International Journal of Oral and Maxillofacillary Surgery 2006;35(7):588‐93. - PubMed
Dimopoulos 2006
-
- Dimopoulos MA, Kastritis E, Anagnostopoulos A, Melakopoulos I, Gika D, Moulopoulos LA, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica 2006;91:968‐71. - PubMed
Drake 2008
Dunford 2001
-
- Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, et al. Structure‐activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen‐containing bisphosphonates. Journal of Pharmacology and Experimental Therapeutics 2001;296(2):235‐42. [PUBMED: 11160603] - PubMed
Durie 2005
-
- Durie BG, Katz M, Crowley J. Osteonecrosis of the jaw and bisphosphonates. New England Journal of Medicine 2005; Vol. 353, issue 1:99‐102.. [PUBMED: 16000365] - PubMed
Egger 2001
-
- Egger M, Davey Smith G, Altman DG. Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd edition. London: BMJ, 2001.
Elad 2006
-
- Elad S, Yarom N, Hamed W, Ayalon S, Yahalom R, Regev E. Osteomylelitis and necrosis of the jaw in patients treated with bisphosphonates: a comparative study focused on multiple myeloma. Clinical and Laboratory Haematology 2006;28(6):393‐8. - PubMed
Ficarra 2005
-
- Ficarra G, Beninati F, Rubino I, Vannucchi A, Longo G, Tonelli P, et al. Osteonecrosis of the jaws in periodontal patients with a history of bisphosphonates treatment. Journal of Clinical Periodontology 2005;32:1123‐8. - PubMed
Fortuna 2012
-
- Fortuna G, Ruoppo E, Pollio A, Aria M, Adamo D, Leuci S, et al. Multiple myeloma vs. breast cancer patients with bisphosphonates‐related osteonecrosis of the jaws: a comparative analysis of response to treatment and predictors of outcome. Journal of Oral Pathology & Medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology 2012;41(3):222‐8. [PUBMED: 22092390] - PubMed
Fromm 1991
-
- Fromm GA, Vega E, Plantalech L, Galich AM, Mautalen CA. Differential action of pamidronate on trabecular and cortical bone in women with involutional osteoporosis. Osteoporosis International 1991;1:126‐33. - PubMed
Gabbert 2015
Gander 2014
-
- Gander T, Obwegeser JA, Zemann W, Gratz KW, Jacobsen C. Malignancy mimicking bisphosphonate‐associated osteonecrosis of the jaw: a case series and literature review. Oral Surgery, Oral Medicine, oral pathology and Oral Radiology 2014;117(1):32‐6. [PUBMED: 24332325] - PubMed
Garcia‐Garay 2006
-
- Garcia‐Garay C, Gonzalez‐Garcia C, Juliana Majado M, Santos T, Borrego D. Osteonecrosis of the jaw in multiple myeloma patients: experience of two hospitals. Blood 2006;108(11):Abstract 5086.
GRADEpro 2008 [Computer program]
-
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro. Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2008.
Greipp 2005
-
- Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. Journal of Clinical oOncology : official journal of the American Society of Clinical Oncology 2005;23(15):3412‐20. [PUBMED: 15809451] - PubMed
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl E, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] - PubMed
Guyatt 2011a
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] - PubMed
Guyatt 2011c
-
- Guyatt G, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines: 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] - PubMed
Guyatt 2011d
-
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] - PubMed
Guyatt 2011e
-
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ risk of bias. Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] - PubMed
Hansen 2006
-
- Hansen T, Kunkel M, Weber A, Kirkpatrick CJ. Osteonecrosis of the jaws in patients treated with bisphosphonates: histomorphologic analysis in comparison with infected osteoradionecrosis. Journal of Oral Pathology & Medicine 2006;35(3):155‐60. - PubMed
Hay 2006
-
- Hay KD, Bishop PA. Association of osteonecrosis of the jaws and bisphosphonate pharmacotherapy: dental implications. New Zealand Dental Journal 2006;102(1):4‐9. - PubMed
Herbozo 2007
-
- Herbozo PJ, Briones DL, Ferres AJ, Torrealba RL. Severe spontaneous cases of bisphosphonate‐related osteonecrosis of the jaws. Journal of Oral and Maxillofacial Surgery 2007;65(8):1650‐4. - PubMed
Higgins 1996
Higgins 2004
-
- Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta‐regression. Statistics in Medicine 2004;23(11):1663‐82. [PUBMED: 15160401] - PubMed
Higgins 2011a
-
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Altman DG, Sterne JAC (editors) on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011d
-
- Jonathan AC Sterne, Matthias Egger and David Moher (editors) on behalf of the Cochrane Bias Methods Group. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org 2011.
Hoff 2006
-
- Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, et al. Osteonecrosis of the jaw in patients receiving intravenous bisphosphonate therapy. Journal of Clinical Oncology 2006;24:8528a.
Junquera 2009
-
- Junquera L, Gallego L, Cuesta P, Pelaz A, Vicente JC. Clinical experiences with bisphosphonate‐associated osteonecrosis of the jaws: analysis of 21 cases. American Journal of Otolaryngology 2009;30(6):390‐5. [PUBMED: 19880027] - PubMed
Jüni 2001
Kademani 2006
-
- Kademani D, Koka S, Lacy MQ, Rajkumar SV. Primary surgical therapy for osteonecrosis of the jaw secondary to bisphosphonate therapy. Mayo Clinic Proceedings 2006;81(8):1100‐3. - PubMed
Kamoh 2012
-
- Kamoh AK, Ogle O. Bisphosphonate‐related osteonecrosis of the jaws‐‐a case report. Compendium of Continuing Education in Dentistry (Jamesburg, N.J. : 1995) 2012;33(5):e74‐7. [PUBMED: 23268588] - PubMed
Katz 2005
-
- Katz H. Endodontic implications of bisphosphonate‐associated osteonecrosis of the jaws: a report of three cases. Journal of Endodontics 2005;31:831‐4. - PubMed
Khamaisi 2006
-
- Khamaisi M, Regev E, Yarom N, Avni B, Leitersdorf E, Raz I, et al. Possible association between diabetes and bisphosphonate‐related jaw osteonecrosis. Journal of Clinical Endocrinology and Metabolism 2007;92(3):1172‐5. - PubMed
Kumar 2007
-
- Kumar V, Pass B, Guttenberg SA, Ludlow J, Emery RW, Tyndall DA, et al. Bisphosphonate‐related osteonecrosis of the jaws: a report of three cases demonstrating variability in outcomes and morbidity. Journal of the American Dental Association 2007;138(5):602‐9. - PubMed
Kut 2004
-
- Kut V, Mehta J, Tariman J, et al. Osteonecrosis of the jaw in myeloma patients receiving pamidronate or zoledronate. Blood 2004;104:Abstract 4933.
Lambert 2005
-
- Lambert PC, Sutton AJ, Burton PR, Abrams KR, Jones DR. How vague is vague? A simulation study of the impact of the use of vague prior distributions in MCMC using WinBUGS. Statistics in Medicine 2005;24(15):2401‐28. [PUBMED: 16015676] - PubMed
Laupacis 1988
-
- Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. New England Journal of Medicine 1988;318:1728‐33. - PubMed
Lazarovici 2009
-
- Lazarovici TS, Yahalom R, Taicher S, Elad S, Hardan I, Yarom N. Bisphosphonate‐related osteonecrosis of the jaws: a single‐center study of 101 patients. Journal of Oral and Maxillofacial Surgery 2009;67(4):850‐5. [PUBMED: 19304045] - PubMed
Lenz 2005
-
- Lenz JH, Steiner‐Krammer B, Schmidt W, Fietkau R, Mueller PC, Gundlach KK. Does avascular necrosis of the jaws in cancer patients only occur following treatment with bisphosphonates?. Jornal of Craniomaxillofacillary Surgery 2005;33:395‐403. - PubMed
Liberati 2009
-
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology 2009;62(10):e1‐34. [PUBMED: 19631507] - PubMed
Lu 2004
Lufkin 1994
-
- Lufkin EG, Argueta R, Whitaker MD, Cameron AL, Wong VH, Egan KS, et al. Pamidronate: an unrecognized problem in gastrointestinal tolerability. Osteoporosis International 1994;4:320‐2. - PubMed
Lugassy 2004
-
- Lugassy G, Shaham R, Nemets A, Ben‐Dor D, Nahlieli O. Severe osteomyelitis of the jaw in long‐term survivors of multiple myeloma: a new clinical entity. American Journal of Medicine 2004;117:440‐1. - PubMed
Magopoulos 2007
-
- Magopoulos C, Karakinaris G, Telioudis Z, Vahtsevanos K, Dimitrakopoulos I, Antoniadis K, et al. Osteonecrosis of the jaws due to bisphosphonate use: a review of 60 cases and treatment proposals. American Journal of Otolaryngology 2007;28(3):158‐63. - PubMed
Marunick 2005
-
- Marunick M, Miller R, Gordon S. Adverse oral sequelae to bisphosphonate administration. Journal of Michigan Dental Association 2005;87:44‐9. - PubMed
Melo 2005
-
- Melo MD, Obeid G. Osteonecrosis of the jaws in patients with a history of receiving bisphosphonate therapy: strategies for prevention and early recognition. Journal of the American Dental Association 2005;136(12):1675‐81. - PubMed
Merigo 2006
-
- Merigo E, Manfredi M, Meleti M, Guidotti R, Ripasarti A, Zanzucchi E, et al. Bone necrosis of the jaws associated with bisphosphonate treatment: a report of twenty‐nine cases. Acta Bio‐medica: Atenei Parmensis 2006;77(2):109‐17. - PubMed
Migliorati 2005
-
- Migliorati CA, Schubert MM, Peterson DE, Seneda LM. Bisphosphonate‐associated osteonecrosis of mandibular and maxillary bone: an emerging oral complication of supportive cancer therapy. Cancer 2005;104:83‐93. - PubMed
Montazeri 2007
-
- Montazeri AH, Erskine JG, McQuaker IG. Oral sodium clodronate induced osteonecrosis of the jaw in a patient with myeloma. European Journal of Haematology 2007;79(1):69‐71. - PubMed
Mortensen 2007
-
- Mortensen M, Lawson W, Montazem A. Osteonecrosis of the jaw associated with bisphosphonate use: Presentation of seven cases and literature review. Laryngoscope 2007;117(1):30‐4. - PubMed
Murad 2007
-
- Murad OM, Arora S, Farag AF, Guber HA. Bisphosphonates and osteonecrosis of the jaw: a retrospective study. Endocrine Practice 2007;13(3):232‐8. - PubMed
Pastor‐Zuazaga 2006
-
- Pastor‐Zuazaga D, Garatea‐Crelgo J, Martino‐Gorbea R, Etayo‐Pérez A, Sebastián‐López C. Osteonecrosis of the jaws and bisphosphonates: report of three cases. Medicina Oral, Patología Oral y Cirugía Bucal 2006;11(1):76‐9. - PubMed
Phal 2007
Pires 2005
-
- Pires FR, Miranda A, Cardoso ES, Cardoso AS, Fregnani ER, Pereira CM, et al. Oral avascular bone necrosis associated with chemotherapy and bisphosphonate therapy. Oral Diseases 2005;11:365‐9. - PubMed
Polizzotto 2006
-
- Polizzotto MN, Cousins V, Schwarer AP. Bisphosphonate‐associated osteonecrosis of the auditory canal. British Journal of Haematology 2006;132(1):114. - PubMed
Pozzi 2007
-
- Pozzi S, Marcheselli R, Sacchi S, Baldini L, Angrilli F, Pennese E, et al. Bisphosphonate‐associated osteonecrosis of the jaw: a review of 35 cases and an evaluation of its frequency in multiple myeloma patients. Leukemia & Lymphoma 2007;48(1):56‐64. - PubMed
Puhan 2014
-
- Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ (Clinical research ed.) 2014;349:g5630. [PUBMED: 25252733] - PubMed
Purcell 2005
-
- Purcell PM, Boyd IW. Bisphosphonates and osteonecrosis of the jaw. Medical Journal of Australia 2005;182:417‐8. - PubMed
Rajkumar 2010
-
- Rajkumar SV. Zoledronic acid in myeloma: MRC Myeloma IX. Lancet 2010;376(9757):1965‐6. [PUBMED: 21131042] - PubMed
RevMan 5.3 [Computer program]
-
- The Nordic Cochrane Centre. Review Manager (RevMan). Version 5.3. Copenhagen: The Cochrane Collaboration, 2014.
Ruggiero 2004
-
- Ruggiero SL, Meherotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. Journal of Oral and Maxillofacillary Surgery 2004;62:527‐34. - PubMed
Saad 2012
-
- Saad F, Brown JE, Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active‐controlled phase III trials in cancer patients with bone metastases. Annals of Oncology : official journal of the European Society for Medical Oncology / ESMO 2012;23(5):1341‐7. [PUBMED: 21986094] - PubMed
Salanti 2011
-
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [PUBMED: 20688472] - PubMed
Salanti 2014
Salesi 2006
-
- Salesi N, Pistilli R, Marcelli V, Govoni FA, Bozza F, Bossone G, et al. Bisphosphonates and oral cavity avascular bone necrosis: a review of twelve cases. Anticancer Research 2006;26(4B):3111‐5. - PubMed
Saussez 2009
-
- Saussez S, Javadian R, Hupin C, Magremanne M, Chantrain G, Loeb I, et al. Bisphosphonate‐related osteonecrosis of the jaw and its associated risk factors: a Belgian case series. Laryngoscope 2009;119(2):323‐9. [PUBMED: 19172621] - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias, dimension of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. - PubMed
Scurrah 2000
-
- Scurrah KJ, Palmer LJ, Burton PR. Variance components analysis for pedigree‐based censored survival data using generalized linear mixed models (GLMMs) and Gibbs sampling in BUGS. Genetic Epidemiology 2000;19(2):127‐48. [PUBMED: 10962474] - PubMed
Senel 2007
-
- Senel FC, Saracoglu Tekin U, Durmus A, Bagis B. Severe osteomyelitis of the mandible associated with the use of non‐nitrogen‐containing bisphosphonate (disodium clodronate): report of a case. Journal of Oral and Maxillofacillary Surgery 2007;65(3):562‐5. - PubMed
Shipman 1997
-
- Shipman CM, Rogers MJ, Apperley JF. Bisphosphonates induce apoptosis in human myeloma cells: a novel anti‐tumour activity. British Journal of Haematology 1997;98:665‐72. - PubMed
Sitters 2005
-
- Sitters MA, Caldwell CS. Bisphosphonates, dental care and osteonecrosis of the jaws. Texas Dental Journal 2005;122:968‐72. - PubMed
STATA V10.1 [Computer program]
-
- StataCorp LP. STATA. Version 10.1. College Station: StataCorp LP, 2008.
Sterne 2001
-
- Sterne JAC, Egger M, Smith GD. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith S, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd edition. London: BMJ Books, 2001:189‐208.
Tanvetyanon 2006
-
- Tanvetyanon T, Stiff PJ. Management of the adverse effects associated with intravenous bisphosphonates. Annals of Oncology 2006;17:897‐907. - PubMed
Tennis 2012
-
- Tennis P, Rothman KJ, Bohn RL, Tan H, Zavras A, Laskarides C, et al. Incidence of osteonecrosis of the jaw among users of bisphosphonates with selected cancers or osteoporosis. Pharmacoepidemiology and Drug Safety 2012;21(8):810‐7. [PUBMED: 22711458] - PubMed
Then 2012
-
- Then C, Horauf N, Otto S, Pautke C, Tresckow E, Rohnisch T, et al. Incidence and risk factors of bisphosphonate‐related osteonecrosis of the jaw in multiple myeloma patients having undergone autologous stem cell transplantation. Onkologie 2012;35(11):658‐64. [PUBMED: 23147542] - PubMed
Thompson 1997
-
- Thompson SG, Smith TC, Sharp SJ. Investigating underlying risk as a source of heterogeneity in meta‐analysis. Statistics in Medicine 1997;16(23):2741‐58. - PubMed
Thompson 2002
-
- Thompson SG, Higgins JP. How should meta‐regression analyses be undertaken and interpreted?. Statistics in Medicine 2002;21(11):1559‐73. [PUBMED: 12111920] - PubMed
Thumbigere‐Math 2012
-
- Thumbigere‐Math V, Tu L, Huckabay S, Dudek AZ, Lunos S, Basi DL, et al. A retrospective study evaluating frequency and risk factors of osteonecrosis of the jaw in 576 cancer patients receiving intravenous bisphosphonates. American Journal of Clinical Oncology 2012;35(4):386‐92. [PUBMED: 22561331] - PubMed
Tierney 2007
Tosi 2006b
-
- Tosi P, Zamagni E, Cangini D, Tacchetti P, Raimondo F, Catalano L, et al. Osteonecrosis of the jaws in newly diagnosed multiple myeloma patients treated with zoledronic acid and thalidomide‐dexamethasone. Blood 2006;108(12):3951‐2. - PubMed
Treister 2006
-
- Treister N, Woo SB. Images in clinical medicine. Bisphosphonate‐associated osteonecrosis of the jaw. New England Journal of Medicine 2006;355(22):2348. - PubMed
Tricot 2000
-
- Tricot G. Multiple myeloma and other plasma cell disorders. In: Hoffman R, Benz EJ, Shattil SJ, et al. editor(s). Hematology: Basic Principles and Practice. 3rd Edition. Philadelphia: Churchill Livingstone, 2000.
Van Holten‐Verzantvoort 1993
-
- Holten‐Verzantvoort AT, Kroon HM, Bijvoet OL, Cleton FJ, Beex LV, Blijham G, et al. Palliative pamidronate treatment in patients with bone metastases from breast cancer. Journal of Clinical Oncology 1993;11:491‐8. - PubMed
Vannucchi 2005
-
- Vannucchi AM, Ficarra G, Antonioli E, Bosi A. Osteonecrosis of the jaw associated with zoledronate therapy in a patient with multiple myeloma. British Journal of Haematology 2005;28:738. - PubMed
Vescovi 2012
-
- Vescovi P, Merigo E, Meleti M, Manfredi M, Guidotti R, Nammour S. Bisphosphonates‐related osteonecrosis of the jaws: a concise review of the literature and a report of a single‐centre experience with 151 patients. Journal of Oral Pathology & Medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology 2012;41(3):214‐21. [PUBMED: 21958312] - PubMed
Walter 2007
-
- Walter C, Grőtz KA, Kunkel M, Al‐Nawas B. Prevalence of bisphosphonate associated osteonecrosis of the jaw within the field of osteonecrosis. Support Care Cancer 2007;15(2):197‐202. - PubMed
Watters 2013
-
- Watters AL, Hansen HJ, Williams T, Chou JF, Riedel E, Halpern J, et al. Intravenous bisphosphonate‐related osteonecrosis of the jaw: long‐term follow‐up of 109 patients. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology 2013;115(2):192‐200. [PUBMED: 23036797] - PubMed
Wickham 2013
-
- Wickham N, Crawford A, Carney AS, Goss AN. Bisphosphonate‐associated osteonecrosis of the external auditory canal. Journal of Laryngology and Otology 2013;127 Suppl 2:S51‐3. [PUBMED: 23673152] - PubMed
Wong 2002
Wutzl 2006
-
- Wutzl A, Eisenmenger G, Hoffmann M, Czerny C, Moser D, Pietschmann P, et al. Osteonecrosis of the jaws and bisphosphonate treatment in cancer patients. Wiener Klinische Wochenschrift 2006;118(15‐16):473‐8. - PubMed
Yeo 2005
-
- Yeo AC, Lye KW, Poon CY. Bisphosphonate‐related osteonecrosis of the jaws. Singapore Dental Journal 2005;27:36‐40. - PubMed
Zarychanski 2006
-
- Zarychanski R, Elphee E, Walton P, Johnston J. Osteonecrosis of the jaw associated with pamidronate therapy. American Journal of Hematology 2006;81:73‐5. - PubMed
Zervas 2006
-
- Zervas K, Verrou E, Teleioudis Z, Vahtsevanos K, Banti A, Mihou D, et al. Incidence, risk factors and management of osteonecrosis of the jaw in patients with multiple myeloma: a single‐centre experience in 303 patients. British Journal of Haematology 2006;134(6):620‐3. - PubMed
References to other published versions of this review
Djulbegovic 2002
Mhaskar 2010
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

