Structure-Guided Synthesis and Mechanistic Studies Reveal Sweetspots on Naphthyl Salicyl Hydrazone Scaffold as Non-Nucleosidic Competitive, Reversible Inhibitors of Human Ribonucleotide Reductase
- PMID: 29253340
- PMCID: PMC5808567
- DOI: 10.1021/acs.jmedchem.7b00530
Structure-Guided Synthesis and Mechanistic Studies Reveal Sweetspots on Naphthyl Salicyl Hydrazone Scaffold as Non-Nucleosidic Competitive, Reversible Inhibitors of Human Ribonucleotide Reductase
Abstract
Ribonucleotide reductase (RR), an established cancer target, is usually inhibited by antimetabolites, which display multiple cross-reactive effects. Recently, we discovered a naphthyl salicyl acyl hydrazone-based inhibitor (NSAH or E-3a) of human RR (hRR) binding at the catalytic site (C-site) and inhibiting hRR reversibly. We herein report the synthesis and biochemical characterization of 25 distinct analogs. We designed each analog through docking to the C-site of hRR based on our 2.7 Å X-ray crystal structure (PDB ID: 5TUS). Broad tolerance to minor structural variations preserving inhibitory potency is observed. E-3f (82% yield) displayed an in vitro IC50 of 5.3 ± 1.8 μM against hRR, making it the most potent in this series. Kinetic assays reveal that E-3a, E-3c, E-3t, and E-3w bind and inhibit hRR through a reversible and competitive mode. Target selectivity toward the R1 subunit of hRR is established, providing a novel way of inhibition of this crucial enzyme.
Conflict of interest statement
The authors declare no competing financial interests
Figures

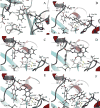

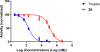


References
-
- Brown NC, Reichard P. Role of effector binding in allosteric control of ribonucleoside diphosphate reductase. J. Mol. Biol. 1969;46:39–55. - PubMed
- Thelander L, Reichard P. Reduction of ribonucleotides. Annu. Rev. Biochem. 1979;48:133–158. - PubMed
- Eriksson M, Uhlin U, Ramaswamy S, Ekberg M, Regnström K, Sjöberg BM, Eklund H. Binding of allosteric effectors to ribonucleotide reductase protein R1: reduction of active-site cysteines promotes substrate binding. Structure. 1997;5:1077–1092. - PubMed
- Larsson KM, Jordan A, Eliasson R, Reichard P, Logan DT, Nordlund P. Structural mechanism of allosteric substrate specificity regulation in a ribonucleotide reductase. Nat. Struct. Mol. Biol. 2004;11:1142–1149. - PubMed
- Xu H, Faber C, Uchiki T, Fairman JW, Racca J, Dealwis CG. Structures of eukaryotic ribonucleotide reductase I provide insights into dNTP regulation. Proc. Natl. Acad. Sci. 2006;103:4022–4027. - PMC - PubMed
- Wang J, Lohman GJ, Stubbe J. Enhanced subunit interactions with gemcitabine-5′-diphosphate inhibit ribonucleotide reductases. Proc. Natl. Acad. Sci. 2007;104:14324–14329. - PMC - PubMed
- Kashlan OB, Cooperman BS. Comprehensive model for allosteric regulation of mammalian ribonucleotide reductase: refinements and consequences. Biochemistry. 2003;42:1696–1706. - PubMed
-
- Rosell R, Crino L, Danenberg K, Scagliotti G, Bepler G, Taron M, Alberola V, Provencio M, Camps C, De MF, Sanchez JJ, Penas R. Targeted therapy in combination with gemcitabine in non-small cell lung cancer. Semin. Oncol. 2003;30:19–25. - PubMed
- Rosell R, Fossella F, Milas L. Molecular markers and targeted therapy with novel agents: prospects in the treatment of non-small cell lung cancer. Lung Cancer. 2002;38:43–49. - PubMed
- Rosell R, Taron M, Sanchez JM, Moran T, Reguart N, Besse B, Isla D, Massuti B, Alberola V, Sanchez JJ. The promise of pharmacogenomics: gemcitabine and pemetrexed. Oncology. 2004;18:70–76. - PubMed
-
- Knappenberger AJ, Ahmad MF, Viswathanan R, Dealwis CG, Harris ME. Nucleotide analog triphosphates allosterically regulate human ribonucleotide reductase and identify chemical determinants that drive substrate specificity. Biochemistry. 2016;55:5884–5896. - PubMed
- Fu Y, Lin H, Wisitpitthaya S, Blessing WA, Aye Y. A fluorometric readout reporting the kinetics of nucleotide-induced human ribonucleotide reductase oligomerization. ChemBioChem. 2014;15:2598–2604. - PMC - PubMed
- Rofougaran R, Vodnala M, Hofer A. Enzymatically active mammalian ribonucleotide reductase exists primarily as an a6b2 octamer. J. Biol. Chem. 2006;281:27705–27711. - PubMed
-
- Ando N, Brignole EJ, Zimanyi CM, Funk MA, Yokoyama K, Asturias FJ, Stubbe J, Drennan CL. Structural interconversions modulate activity of Escherichia coli ribonucleotide reductase. Proc. Natl. Acad. Sci. U.S.A. 2011;108:21046–21051. - PMC - PubMed
- Ando N, Li H, Brignole EJ, Thompson S, McLaughlin MI, Page JE, Asturias FJ, Stubbe J, Drennan CL. Allosteric inhibition of human ribonucleotide reductase by dATP entails the stabilization of a hexamer. Biochemistry. 2016;55:373–381. - PMC - PubMed
- Zimanyi CM, Chen PYT, Kang G, Funk MA, Drennan CL. Molecular basis for allosteric specificity regulation in class IA ribonucleotide reductase from Escherichia coli. eLife. 2016;5:e07141. - PMC - PubMed
- Wisitpitthaya S, Zhao Y, Long MJ, Li M, Fletcher EA, Blessing WA, Weiss RS, Aye Y. Cladribine and fludarabine nucleotides induce distinct hexamers defining a common mode of reversible RNR inhibition. ACS Chem. Biol. 2016;11:2021–2032. - PMC - PubMed
-
- Ahmad MF, Dealwis CG. The structural basis for the allosteric regulation of ribonucleotide reductase. In: Giraldo J, Ciruela F, editors. Oligomerization in Health and Disease. Vol. 117. Academic Press; Cambridge, MA: 2013. pp. 389–410. - PMC - PubMed
- Cooperman BS, Dealwis CG. Allosteric regulation and inhibition of class 1a ribonucleotide reductase activity. In: Anderson K, editor. Riboncleotide Reductase. Nova Science Publishers, Inc; Hauppauge, NY: 2008. pp. 99–124. Ch. 4.
- Fairman JW, Wijerathna SR, Ahmad MF, Xu H, Nakano R, Jha S, Prendergast J, Welin RM, Flodin S, Roos A, Nordlund P, Li Z, Walz T, Dealwis CG. Structural basis for allosteric regulation of human ribonucleotide reductase by nucleotide-induced oligomerization. Nat. Struct. Mol. Biol. 2011;18:316–322. - PMC - PubMed
- Xu H, Fairman JW, Wijerathna SR, Kreischer NR, LaMacchia J, Helmbrecht E, Cooperman BS, Dealwis CG. The structural basis for peptidomimetic inhibition of eukaryotic ribonucleotide reductase: A conformationally flexible pharmacophore. J. Med. Chem. 2008;51:4653–4659. - PMC - PubMed
- Aye Y, Li M, Long MJ, Weiss RS. Ribonucleotide reductase and cancer: biological mechanisms and targeted therapies. Oncogene. 2015;34:2011–2021. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

