On the utility of a compartmental population kinetics model of intestinal epithelial stem cell proliferation and differentiation
- PMID: 29254493
- PMCID: PMC5735948
- DOI: 10.1186/s12976-017-0071-8
On the utility of a compartmental population kinetics model of intestinal epithelial stem cell proliferation and differentiation
Abstract
Background: The small intestinal epithelium is a dynamic system with specialized cell types. The various cell populations of this tissue are continually renewed and replenished from stem cells that reside in the small intestinal crypt. The cell types and their locations in the crypt and villus are well known, but the details of the kinetics of stem cell division, and precursor cell proliferation and differentiation into mature enterocytes and secretory cells are still being studied. These proliferation and differentiation events have been extensively modeled with a variety of computational approaches in the past.
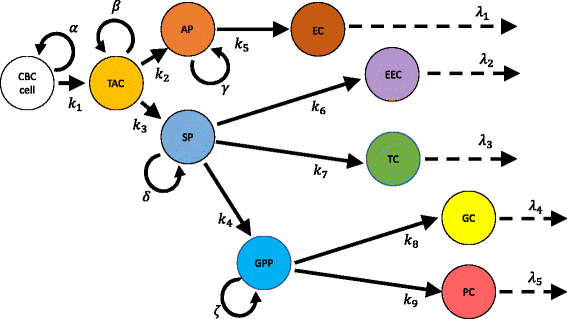
Methods: A compartmental population kinetics model, incorporating experimentally measured proliferation rates for various intestinal epithelial cell types, is implemented for a previously reported scheme for the intestinal cell dynamics. A sensitivity analysis is performed to determine the effect that varying the model parameters has upon the model outputs, the steady-state cell populations.
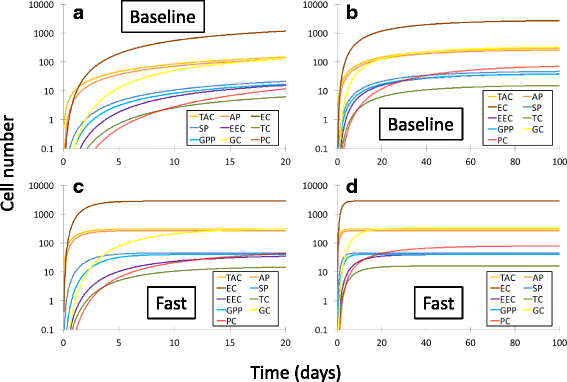
Results: The model is unable to reproduce the experimentally known timescale of renewal of the intestinal epithelium if literature values for the proliferation rates of stem cells and transit amplifying cells are employed. Unphysically large rates of proliferation result when these parameters are allowed to vary to reproduce this timescale and the steady-state populations of terminally differentiated intestinal epithelial cells. Sensitivity analysis reveals that the strongest contributor to the steady-state populations is the transit amplifying cell proliferation rate when literature values are used, but that the differentiation rate of transit amplifying cells to secretory progenitor cells dominates when all parameters are allowed to vary.
Conclusions: A compartmental population kinetics model of proliferation and differentiation of cells of the intestinal epithelium can provide a simplifying means of understanding a complicated multistep process. However, when literature values for proliferation rates of the crypt based columnar and transit amplifying cell populations are employed in the model, it cannot reproduce the experimentally known timescale of intestinal epithelial renewal. Nevertheless, it remains a valuable conceptual tool, and its sensitivity analysis provides important clues for which events in the process are the most important in controlling the steady-state populations of specialized intestinal epithelial cells.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable, no human subjects.
Consent for publication
Not applicable, no human subjects.
Competing interests
The author declares that he/she has no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Wnt control of stem cells and differentiation in the intestinal epithelium.Exp Cell Res. 2005 Jun 10;306(2):357-63. doi: 10.1016/j.yexcr.2005.02.022. Epub 2005 Apr 7. Exp Cell Res. 2005. PMID: 15925592 Review.
-
Evolution of pig intestinal stem cells from birth to weaning.Animal. 2019 Dec;13(12):2830-2839. doi: 10.1017/S1751731119001319. Epub 2019 Jun 14. Animal. 2019. PMID: 31199215
-
Stem cells, self-renewal, and differentiation in the intestinal epithelium.Annu Rev Physiol. 2009;71:241-60. doi: 10.1146/annurev.physiol.010908.163145. Annu Rev Physiol. 2009. PMID: 18808327 Review.
-
Obesity, independent of diet, drives lasting effects on intestinal epithelial stem cell proliferation in mice.Exp Biol Med (Maywood). 2018 Jun;243(10):826-835. doi: 10.1177/1535370218777762. Exp Biol Med (Maywood). 2018. PMID: 29932373 Free PMC article.
-
Canonical Wnt signals combined with suppressed TGFβ/BMP pathways promote renewal of the native human colonic epithelium.Gut. 2014 Apr;63(4):610-21. doi: 10.1136/gutjnl-2012-304067. Epub 2013 Jul 5. Gut. 2014. PMID: 23831735 Free PMC article.
Cited by
-
The HSP90/R2TP assembly chaperone promotes cell proliferation in the intestinal epithelium.Nat Commun. 2021 Aug 10;12(1):4810. doi: 10.1038/s41467-021-24792-4. Nat Commun. 2021. PMID: 34376666 Free PMC article.
-
Chronic TNFα-driven injury delays cell migration to villi in the intestinal epithelium.J R Soc Interface. 2018 Aug;15(145):20180037. doi: 10.1098/rsif.2018.0037. J R Soc Interface. 2018. PMID: 30068555 Free PMC article.
-
Identification of influential proteins in the classical retinoic acid signaling pathway.Theor Biol Med Model. 2018 Oct 16;15(1):16. doi: 10.1186/s12976-018-0088-7. Theor Biol Med Model. 2018. PMID: 30322383 Free PMC article.
-
Systems Modeling to Quantify Safety Risks in Early Drug Development: Using Bifurcation Analysis and Agent-Based Modeling as Examples.AAPS J. 2021 May 20;23(4):77. doi: 10.1208/s12248-021-00580-2. AAPS J. 2021. PMID: 34018069 Free PMC article. Review.
References
-
- Gregorieff A, Stange DE, Kujala P, Begthel H, Van den Born M, Korving J, Peters PJ, Clevers H. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology. 2009;137(4):1333–1345. doi: 10.1053/j.gastro.2009.06.044. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

