Estrogen exposure overrides the masculinizing effect of elevated temperature by a downregulation of the key genes implicated in sexual differentiation in a fish with mixed genetic and environmental sex determination
- PMID: 29254503
- PMCID: PMC5735924
- DOI: 10.1186/s12864-017-4345-7
Estrogen exposure overrides the masculinizing effect of elevated temperature by a downregulation of the key genes implicated in sexual differentiation in a fish with mixed genetic and environmental sex determination
Abstract
Background: Understanding the consequences of thermal and chemical variations in aquatic habitats is of importance in a scenario of global change. In ecology, the sex ratio is a major population demographic parameter. So far, research that measured environmental perturbations on fish sex ratios has usually involved a few model species with a strong genetic basis of sex determination, and focused on the study of juvenile or adult gonads. However, the underlying mechanisms at the time of gender commitment are poorly understood. In an effort to elucidate the mechanisms driving sex differentiation, here we used the European sea bass, a fish species where genetics and environment (temperature) contribute equally to sex determination.
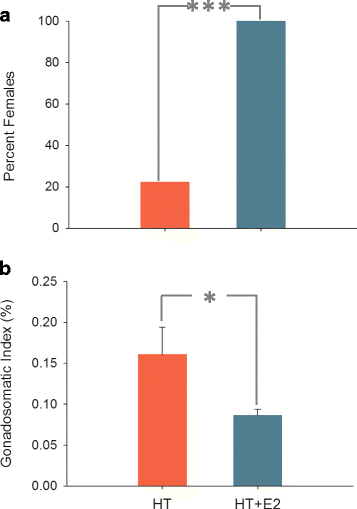
Results: Here, we analyzed the transcriptome of developing gonads experiencing either testis or ovarian differentiation as a result of thermal and/or exogenous estrogen influences. These external insults elicited different responses. Thus, while elevated temperature masculinized genetic females, estrogen exposure was able to override thermal effects and resulted in an all-female population. A total of 383 genes were differentially expressed, with an overall downregulation in the expression of genes involved in both in testicular and ovarian differentiation when fish were exposed to Estradiol-17ß through a shutdown of the first steps of steroidogenesis. However, once the female phenotype was imposed, gonads could continue their normal development, even taking into account that some of the resulting females were fish that otherwise would have developed as males.
Conclusions: The data on the underlying mechanisms operating at the molecular level presented here contribute to a better understanding of the sex ratio response of fish species subjected to a combination of two of the most common environmental perturbations and can have implications in future conservational policies.
Keywords: Climate change; Ecotoxicology; Estradiol; Fish; Phenotypic plasticity; Sex differentiation; Sex ratio; Temperature increase; Transcriptomics.
Conflict of interest statement
Ethics approval and consent to participate
Our facilities are approved for animal experimentation by the Ministry of Agriculture and Fisheries (certificate number 08039–46–A) in accordance with the Spanish law (R.D. 223 of March 1988). As stated in the Methods section, fish used in this experiment were treated in agreement with the European Convention for the Protection of Animals used for Experimental and Scientific Purposes (EST Nu 123, 01/01/91). The experimental protocol was approved by the Spanish National Research Council (CSIC) Ethics Committee within the project AGL2013–41047-R. Fish were sacrificed using an overdose of 2-phenoxyethanol (2PE).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Werren JH, Beukeboom LW. Sex determination, sex ratios, and genetic conflict. Annu Rev Ecol Syst. 1998;29:233–261. doi: 10.1146/annurev.ecolsys.29.1.233. - DOI
-
- Penman DJ, Piferrer F. Fish gonadogenesis. Part 1. Genetic and environmental mechanisms of sex determination. Rev Fish Sci. 2008;16(S1):16–34. doi: 10.1080/10641260802324610. - DOI
-
- Piferrer F, Guiguen G. Fish Gonadogenesis. Part II: molecular biology and genomics of sex differentiation. Rev Fish Sci. 2008;16(S1):35–55. doi: 10.1080/10641260802324644. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- BES-2007-14273/Government of Spain/International
- AGL2010-15939/Government of Spain/International
- Epigen-Aqua contract from (AGL2010-15939)/Government of Spain/International
- CDS2007-002/Spanish Ministry of Economy and Competitiveness/International
- AGL2013-41047-R/Spanish Ministry of Economy and Competitiveness/International
LinkOut - more resources
Full Text Sources
Other Literature Sources

